Gene Therapy Plasmid DNA Preparation
Inquiry
High-quality plasmid DNA is a key component of the gene therapy process. The purity of the plasmid will seriously affect the efficiency of transfection. CD Formulation continues to optimize the plasmid DNA preparation process and constantly provides reliable plasmid DNA preparation services to meet the needs of researchers for high-quality plasmid DNA. Our goal is to work with researchers to promote the progress of gene therapy research and provide more possibilities for gene therapy.
Why Conduct Gene Therapy Plasmid DNA Preparation?
Plasmid DNA (pDNA) is a key component of gene therapy production. Therefore, it is necessary to optimize production to meet gene therapy products' quantity and quality requirements. However, plasmid DNA faces several challenges due to its large size, high cleavage sensitivity, high viscosity, and similarity of impurities in its production process. Therefore, optimizing the preparation process of plasmid DNA is important for the study of plasmid DNA in gene therapy. Optimizing plasmid DNA preparation is better as a gene therapy tool and important for all aspects of production, safety and market application.
Our Services for Gene Therapy Plasmid DNA Preparation
Fermentation
The characterized cell bank is used to prepare pre-cultures for subsequent cultivation in the bioreactor. Here, different operating parameters will be monitored and adjusted, including pH, dissolved oxygen, specific growth rates, limiting substrates, etc.
Cell harvesting and lysis
Use batch processing or limited sedimentation centrifugation to separate biomass produced by fermentation from liquid media. Testing for plasmid integrity, homology, and content is necessary for the biomass. Alkaline lysis is the method used to lyse suspension cells. The plasmid DNA remains in solution following the neutralization reaction. It can be separated from flocculent material, such as proteins and chromosomal DNA, by primary filtration or centrifugation. The supernatant is then filtered once more until the large-scale lysis process. After filtering to get rid of bacteria, the plasmid containing the cleared lysate is treated further on.
Chromatography
Specific chromatographic chromatography techniques are used to separate plasmid DNA from other components of the host cell, which include chromosomal DNA, RNA, nucleotides, lipids, and others. The operating procedure for chromatography chromatography depends on the quality level required for the plasmid product. Our advanced manufacturing process not only removes chromosomal DNA but also unwanted open-loop and linear plasmids.
Formulation
Through downstream processing, purified plasmid DNA is acquired in bulk. Prior to further processing, storage, and application, it must be proportionately treated with the proper buffers or solutions.
Quality control
We also set up a number of quality control procedures since product purity is the assurance of both product quality and safety.
Our Services for Gene Therapy Plasmid DNA Purification
The best solution for plasmid purification is to have a robust and effective overall process in which each step is well-optimized. Currently, the classical three-step chromatography method is more widely used in the market. We have optimized each step of the chromatography process by combining different principles of chromatographic purification techniques and suitable chromatographic media and conducted experimental application studies, which ensures that the process is more stable and efficient based on high purity. The plasmid purification methods we offer are robust, effective, and practical. With the continuous development of the process, we will also continue to optimize the process to help customers improve production efficiency, reduce production costs, and effectively contribute to gene therapy.
- Captures plasmid DNA and removes RNA.
- Removal of open circular DNA (ocDNA).
- Removal of trace impurities and endotoxins.
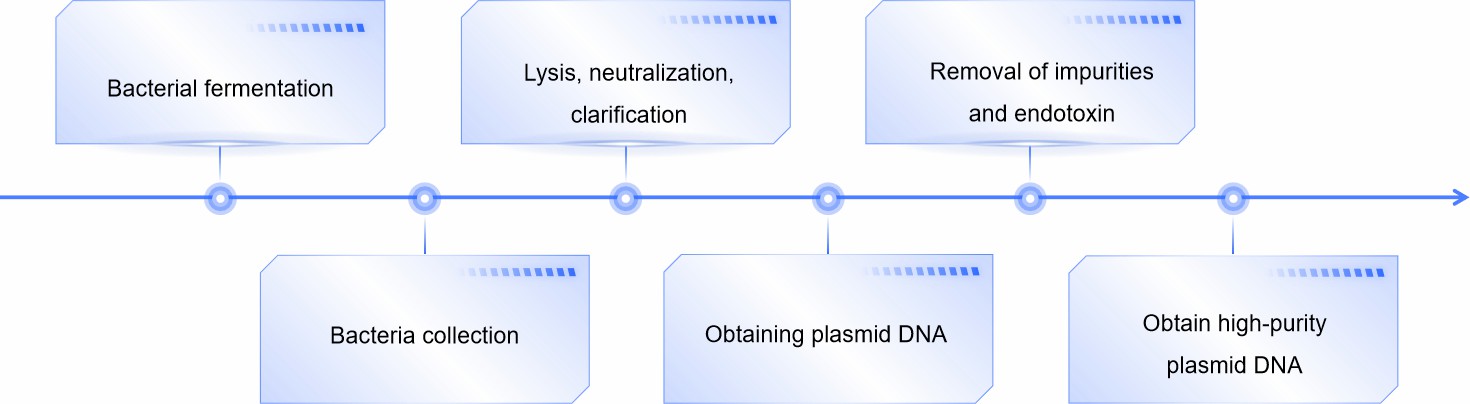 Fig.2 Plasmid DNA acquisition process. (CD Formulation)
Fig.2 Plasmid DNA acquisition process. (CD Formulation)
Our Platforms for Gene Therapy Plasmid DNA Preparation
| Platforms & Technologies |
Content Description |
| Chromatography |
We use specific chromatographic chromatography techniques to separate plasmid DNA from other components of the host cell, including chromosomal DNA, RNA, nucleotides, lipids, residual proteins, amino acids, sugars, and buffer components. |
| Storage of plasmid DNA |
Specific storage system evaluation studies were carried out to assess optimal storage conditions. Preserved plasmid DNA is regularly quantified using capillary gel electrophoresis (CGE), and the results suggest that the DNA molecule is well stabilized. |
| Plasmid DNA quality control |
During the production of plasmid DNA, we strictly control the ratio of superhelical conformation, host DNA residue, and host protein content to ensure that the product quality meets high standards. |
Highlights of Our Gene Therapy Plasmid DNA Preparation
- Full range of plasmid extraction grades. We provide research grade, transfection grade, and other levels of plasmid extraction to meet the needs of different customers.
- Strict quality control. We provide data including appearance, A260/280, superhelix ratio, and so on. We can also provide restriction enzyme analysis, sequencing validation, endotoxin level detection, and other services according to customer needs.
- Flexible range of options. We can provide high-copy or low-copy plasmids for customers' selection according to their requirements.
- Fast delivery time. Our experimental efficiency is high, with medium-volume extraction taking only a few working days.
Published Data
Technology: Plasmid DNA-loaded nanoliposomes development
Journal: Drug Deliv Transl Res
IF: 5.7
Published: 2022
The aim of this study was to develop a simple and novel method to prepare plasmid DNA-loaded nanoliposomes for cancer gene therapy. Murine interleukin-12 (mIL-12) pDNA-loaded nanoliposomes were prepared via novel freeze-drying of a monophase solution method. The physicochemical characteristics, cytotoxicity, and transfection efficiency of the prepared nanoliposomes in murine CT-26 colon carcinoma cells were evaluated. Furthermore, tumor progression and survival rate in CT-26 colon carcinoma-bearing BALB/c mice subsequent to direct intratumoral injections were investigated over a period of 40 days. Using this preparation method, nanoliposomes with particle size of around 300 nm and zeta potential of 96.5 mV were obtained.
CD Formulation has always been at the forefront of gene therapy research, and we continue to optimize our gene therapy formulation process development, aiming to work with researchers to advance gene therapy research. If you are interested in us, please feel free to contact us.
References
- Baradaran B, et al. A novel method for the development of plasmid DNA-loaded nanoliposomes for cancer gene therapy. Drug Deliv Transl Res. 2022 Jun;12(6):1508-1520.


 Fig.2 Plasmid DNA acquisition process. (CD Formulation)
Fig.2 Plasmid DNA acquisition process. (CD Formulation)