Gene Therapy Formulation Studies
Inquiry
Gene therapy is a revolutionary medical approach designed to treat or prevent disease by introducing normal or modified genetic material into a patient's body. Formulation studies in gene therapy is a multi-step process aimed at designing and optimizing therapeutics that can safely and effectively deliver therapeutic genes to target cells. The design and studies of stable formulations play an important role in gene therapy.
CD Formulation is an industry leader in gene therapy formulation studies, and we are committed to providing a range of formulation studies services for gene therapy formulations that increase the targeting, stability and safety of gene delivery. Our experienced research team can provide professional technical support and solutions for your project research and help you optimize your gene therapy formulations.
Importance of Gene Therapy Formulation Studies
- Stability. Gene therapy formulations must be stable during storage to ensure that they remain effective when used. A good formulation will prevent degradation of the gene and delivery vehicle and maintain biological activity.
- Biological distribution. For gene therapy to work, the gene needs to be successfully delivered to the target cells. The formulation can influence the distribution, bioavailability, and targeting of the gene in the body, ensuring that the gene reaches the affected tissue or organ.
- Delivery system. The gene itself usually needs to be combined with a delivery vehicle, such as liposomes, nanoparticles, or viral vectors. The formulation of these vectors needs to be optimized to ensure effective gene delivery while reducing immune reactions or other adverse effects.
- Immunogenicity and safety. Both the gene itself and the vector used have the potential to trigger an immune response. By optimizing the formulation, these immune responses can be attenuated and the safety of gene therapy can be improved.
Explore Our Gene Therapy Formulation Studies
We provide high-quality and reliable gene therapy formulation studies services, including but not limited to cationic liposome formulations, and nanoparticle formulations. Our expert team has many years of service experience in formulation studies, and we can design the best formulation solution according to the customer's project needs to help your project research. Our gene therapy formulation studies services include but are not limited to the following.
Selection and optimization of delivery systems
This process deals with the selection and optimization of viral vectors such as adeno-associated viruses, lentiviruses, adenoviruses, and other vectors. The selection of non-viral vectors, such as liposomes and polymeric nanoparticles, can be targeted according to the experimental objectives.
Stability studies
When making stability selections for gene therapy formulations, we focus on physical stability, i.e., ensuring that gene therapy formulations are stable under varying conditions of temperature, humidity, and light. As well as chemical stability, i.e. preventing degradation of active ingredients or adverse chemical reactions. Colleague biostability studies are also a very important aspect, where we need to ensure the protection of the genetic material from degradation enzymes in vivo.
Dosage design and optimization
We need to select the appropriate route of administration (e.g., intravenous, intramuscular, local injection, etc.) to ensure delivery efficiency and targeting. At the same time, we also need to determine the dose of the drug under different routes of administration, i.e., the concentration and volume need to be strictly controlled.
Excipient selection
We need to determine the buffer solution to keep the pH of the solution stable. Stabilizers also need to be identified to prevent degradation of the formulation during storage and delivery. In addition, protective agents and surfactants are also important, such as coenzymes and PEG (polyethylene glycol), which help to improve the stability and biocompatibility of the genetic formulation.
Quality control and testing
During formulation studies, we need to perform purity testing, sterility testing to ensure that the formulation is free from microbial contamination, endotoxin testing, and many other tests.
Efficacy determination
Efficacy is a key attribute for product characterization, and we need to perform efficacy determination using matrix methods, including direct and indirect determination.
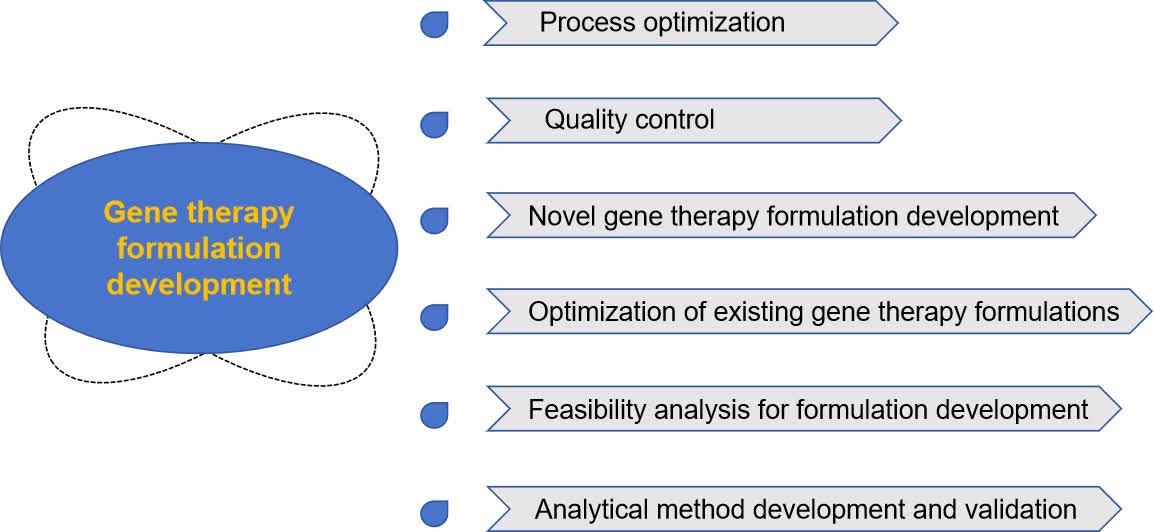 Fig.2 Role of gene therapy formulation studies. (CD Formulation)
Fig.2 Role of gene therapy formulation studies. (CD Formulation)
Applications of Gene Therapy Formulation Studies
- Genetic diseases. Gene therapy has shown great potential in treating certain genetic diseases such as cystic fibrosis and hemophilia.
- Cancer therapy. Recognizing and attacking cancer cells by modifying the patient's T-cells, i.e. CAR-T cell therapy.
- Rare disease therapy. For those rare diseases that lack effective treatments, gene therapy offers new avenues of treatment.
- Ocular diseases. Certain inherited retinal disorders (e.g., Leber congenital amaurosis) have had gene therapy products approved that have shown prolonged efficacy.
Our Platforms for Gene Therapy Formulation Studies
| Platforms & Technologies |
Content Description |
| Encapsulation and protection technology platforms |
We utilize PEGylation for masking immunogenicity and extending half-life in vivo. We also utilize a variety of nanocarriers, such as gold nanoparticles, silica nanoparticles, exosomes, etc., for the protection and efficient transportation of gene materials. |
| Gene delivery technology platforms |
We have established electroporation technology, in which the cell membrane is briefly opened by electrical stimulation to allow the entry of gene materials into the cell. There is also ultrasound technology, which introduces genetic material into cells by utilizing sound waves to permeate the cell membrane. |
| Cell-specific delivery technologies |
Enhances the efficiency of targeted delivery by engaging ligands for specific cellular receptors on the surface of the delivery system. As well as utilized to direct vectors to specific cells or tissues to improve therapeutic targeting and efficiency. |
Highlights of Our Gene Therapy Formulation Studies
- Traceability. We have complete operation records and a review of all experimental processes.
- Cooperate with audits. Our quality assurance department supervises the whole process and can assist the reporting party in being audited for the relevant services after the project is completed.
- Professional team. We have a professional compliance testing service team to provide a full range of services from project management to experimental arrangements.
- Advanced studies platform. We have CGMP-compliant analytical laboratories and manufacturing facilities.
Published Data
Technology: Viral vector manufacturing
Journal: J Pharm Sci
IF: 3.8
Published: 2021
This study comprehensively analyzes the upstream, downstream, and formulation unit operational challenges encountered during AAV vector manufacturing. It discusses how to maintain desired product quality attributes throughout the product shelf life by understanding degradation mechanisms and formulation strategies. Various mechanisms of physical and chemical instability that may be encountered in viral vectors during production and shelf life due to various stress conditions such as heat, shear, freeze-thaw, and light are highlighted. The effects of buffers, pH, excipients, and impurities on the stability of viral vectors are also discussed.
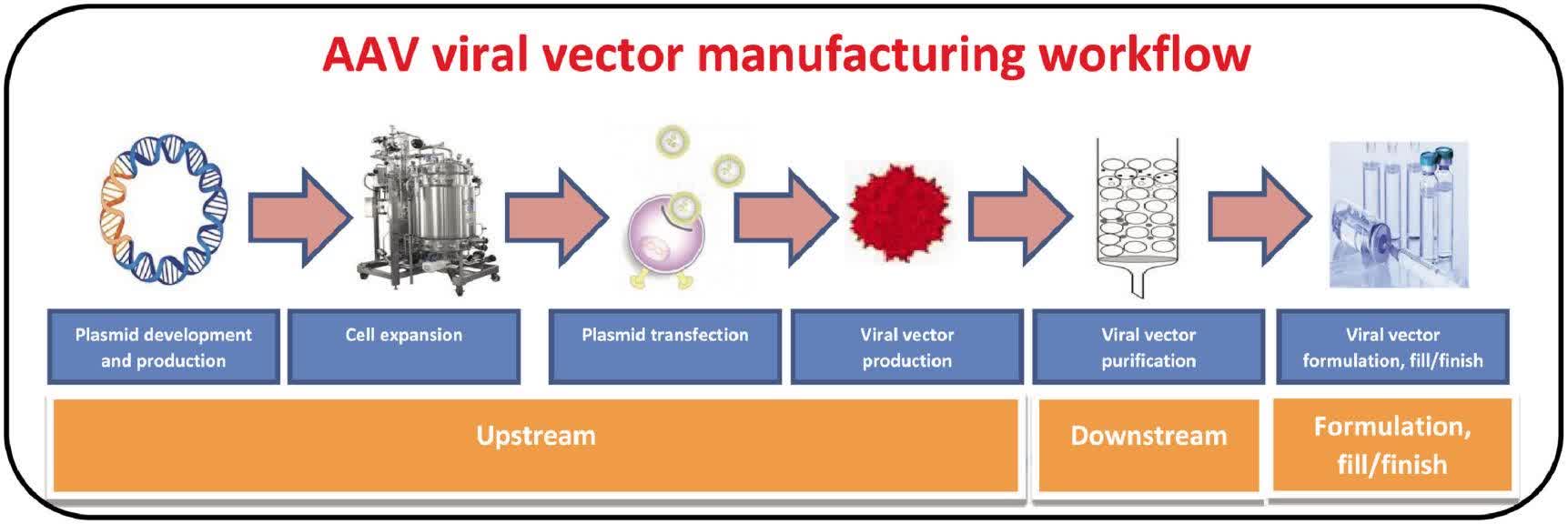 Fig.2 Viral vector manufacturing process workflow. (Srivastava A, et al., 2021)
Fig.2 Viral vector manufacturing process workflow. (Srivastava A, et al., 2021)
CD Formulation is committed to assisting you with your gene therapy formulation research to support your efforts to advance the studies and application of gene therapy formulations. If you are interested in us, please feel free to contact us.
References
- Srivastava A, et al. Manufacturing Challenges and Rational Formulation studies for AAV Viral Vectors. J Pharm Sci. 2021, 110(7):2609-2624.
Related Services


 Fig.2 Role of gene therapy formulation studies. (CD Formulation)
Fig.2 Role of gene therapy formulation studies. (CD Formulation) Fig.2 Viral vector manufacturing process workflow. (Srivastava A, et al., 2021)
Fig.2 Viral vector manufacturing process workflow. (Srivastava A, et al., 2021)