Gene Therapy Formulation Polyethylenimine Residue Testing
Inquiry
Residues of the transfection agent polyethylenimine (PEI), a widely used cationic polymer transfection vector for transient gene expression for the production of recombinant proteins in gene therapy as well as in viral vector production, are of particular concern in gene therapy formulations. Despite the advantages of high transfection efficiency and easy escape from lysosomes, PEI residues may pose a potential risk to patients, as PEI is not readily biodegradable in vivo and is somewhat toxic. CD Formulation has extensive experience in providing services for gene therapy formulation development and quality control testing. Our state-of-the-art testing platform is capable of providing comprehensive and accurate testing for residues, including PEI, and thus plays an important role in ensuring the safety and efficacy of gene therapy products.
The Importance of Gene Therapy Formulation Polyethylenimine Residue Testing
PEI is very easy to bind negatively charged DNA molecules, form complexes and transfect into adherent or suspension cells, which are widely used in the field of gene therapy formulations. However, PEI is not easily biodegradable in vivo and becomes enriched, so it is necessary to strictly control PEI residues in gene therapy formulations to avoid potential risks to organisms.
Explore Our Gene Therapy Formulation Polyethylenimine Residue Testing
Highly sensitive detection method
We use high-performance liquid chromatography with evaporative light scattering detector (HPLC-ELSD), a method suitable for the analysis of semivolatile and non-volatile compounds that cannot be measured by UV detector, especially for PEIs that lack UV-absorbing groups and are not volatile. This method can accurately analyze the residues of transfectants PEIs in the formulations quantitatively, with a high sensitivity and good reproducibility.
Sample pretreatment
Before sample analysis, we dilute and treat the samples appropriately to eliminate matrix interference and improve the recovery of PEI in the samples. For example, the use of a mixture of acidic organic reagents and water as a sample pretreatment reagent can effectively eliminate matrix interference and improve PEI recovery.
Process optimization
During the production of viral vectors, we reduce PEI residues by optimizing the process, such as improving the virus purification step, reducing the amount of PEI used, and adopting more effective removal and purification techniques.
Quality control monitoring
PEI residues are monitored at different stages of the production process to ensure that each step meets predetermined quality standards. This includes testing for PEI residues during clarification, chromatographic purification, and ultrafiltration concentration steps of viral vector production.
Our Technologies for Gene Therapy Formulation Polyethylenimine Residue Testing
When choosing the residue detection method for PEI transfection reagents in gene therapy formulations, we need to choose the appropriate PEI transfection conditions according to the experimental requirements and transfected cell types before using PEI for transfection, and strictly follow our specific experimental procedures to ensure the accuracy of the test results and avoid the interference of PEI residue on the experimental results.
|
Platforms & Technologies |
Content Description |
| High-performance liquid chromatography (HPLC) |
By optimizing the chromatographic conditions, such as selecting the appropriate mobile phase, and adjusting the flow rate and column temperature, the efficient separation and accurate determination of PEI can be achieved. Meanwhile, the accuracy and reliability of detection can be further improved by utilizing methods such as the standard curve or internal standard method. |
| Evaporative light scattering detector (ELSD) |
When standards are not available or the structural characteristics of the compounds are unknown, this approach can identify compounds that do not include chromophores. In addition, the response of ELSD is not dependent on the sample's optical properties, and any sample that is less volatile than the mobile phase can be detected regardless of its functional groups. This characteristic enables PEIs that are not volatile by a UV detector and do not have UV-absorbing groups to be identified using HPLC-ELSD. |
Highlights of Our Gene Therapy Formulation Polyethylenimine Residue Testing
- We have a research and technical team with rich expertise and experience, constantly optimizing detection methods and experimental procedures, aiming to help clients accurately detect PEI residues in gene therapy formulations.
- We ensure high reproducibility and accuracy of test results, in addition to ensuring that the experimental process complies with relevant regulations to ensure service compliance.
- We have an advanced technology platform characterized by high sensitivity and wide linear range detection, which can accurately detect PEI residues at low concentrations in gene therapy formulations.
- We have specialized columns and detectors to continuously optimize the detection process and improve detection efficiency.
Published Data
Technology: Polyethylenimine for gene delivery
Journal: J Funct Biomater
IF: 5.0
Published: 2022
Nanotechnology advancements have led to the creation of various polymer-based drug delivery systems for medical use, especially in cancer treatment. These systems, known for their biocompatibility, can effectively target tumor sites and can be combined with other therapeutic agents for cancer diagnosis and treatment. Polyethyleneimine (PEI), a commonly researched polymer due to its structure and solubility, has been a focus for drug and gene delivery. This article reviews PEI surface modifications to improve its compatibility and functionality, the synthesis of PEI-based nanoparticles, and their applications in cancer therapy and imaging, as well as the challenges ahead.
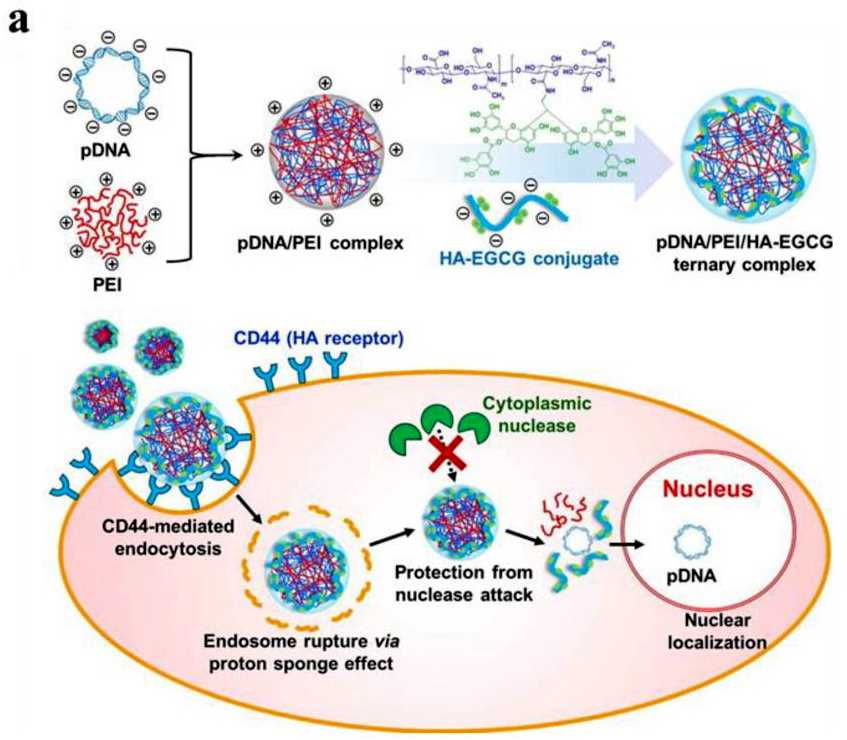 Fig.1 The synthesis and mechanism of self-assembled complexes for gene delivery. (Zhao C, Zhou B. 2022)
Fig.1 The synthesis and mechanism of self-assembled complexes for gene delivery. (Zhao C, Zhou B. 2022)
CD Formulation performs comprehensive, accurate testing of residues, including PEI, to ensure the safety and efficacy of gene therapy formulations. We ensure the reliability and accuracy of our tests and look forward to working with you. If you are interested in us, please feel free to contact us.
References
- Zhao C, Zhou B. Polyethyleneimine-Based Drug Delivery Systems for Cancer Theranostics. J Funct Biomater. 2022, 14(1):12.
Related Services

 Fig.1 The synthesis and mechanism of self-assembled complexes for gene delivery. (Zhao C, Zhou B. 2022)
Fig.1 The synthesis and mechanism of self-assembled complexes for gene delivery. (Zhao C, Zhou B. 2022)