Gene Therapy Formulation Plasmid DNA Residue Testing
Inquiry
Plasmid DNA residue testing focuses on plasmid DNA that may remain in the final product when plasmid DNA is used as a vector or template in the preparation of a gene therapy product. The residual plasmid DNA may originate from the transfection step of the manufacturing process, where the plasmid is introduced into the host cell for the production of a therapeutic protein or viral vector.
CD Formulation is an industry leader in the development of gene therapy formulations, and we rely on our advanced technology platforms and experienced team of expert technicians to provide additional rapid analytical solutions for gene therapy formulation development and manufacturing. We offer plasmid DNA residue testing to determine that the final product does not contain plasmid DNA that is harmful to patients, such as the presence of antibiotic resistance genes or other non-therapeutic sequences.
The Need for Gene Therapy Formulation Plasmid DNA Residue Testing
- Safety guarantee. Residues of plasmid DNA, as a carrier in gene therapy, may trigger an immune response in patients, increase the risk of inflammation, and may even lead to gene mutation or activation of oncogenes, thus posing a safety risk. Therefore, testing for residues can help ensure the safety of therapeutic products in treating patients.
- Regulatory compliance. Drug regulatory authorities in various countries have strict limits on the amount of plasmid DNA residues in gene therapy formulations, and we can ensure that gene therapy formulations comply with the relevant requirements through plasmid DNA residue testing.
- Quality control. Plasmid DNA residue testing helps to monitor the production process to ensure the quality and consistency of biologics. The test can assess whether the purification steps in the production process are effective.
- Optimize the production process. Detection of residual DNA allows for optimization of the production process, such as adjusting lysis conditions, purification steps and parameters to improve product quality and reduce production costs.
Explore Our Gene Therapy Formulation Plasmid DNA Residue Testing
We usually use quantitative polymerase chain reaction (qPCR) for plasmid DNA residue testing and design specific primers and probes to quantify the amount of a particular plasmid sequence. In addition, we also use other techniques, some of which are listed below.
Quantitative reverse transcription polymerase chain reaction (RT-qPCR)
Using particular primer and probe designs, this extremely sensitive quantitative approach enables accurate identification and quantification of plasmid DNA residues in samples. RT-qPCR procedures encompass a range of methodologies, including the TaqMan probe method and the use of certain dyes. These techniques guarantee the assay's specificity since they can identify distinct DNA sequences and detect incredibly low DNA quantities.
Capillary gel electrophoresis (cGE)
CGE combined with a laser-induced fluorescence (LIF) detector can be used to rapidly detect the fragment size distribution of plasmid DNA. This method provides detailed information about the size of the DNA fragments and helps to assess the risk level of residual DNA.
DNA probe hybridization
This method uses a specific DNA probe to hybridize with the target DNA sequence and quantifies the residual DNA by detecting the hybridization signal.
Fluorescent staining method
This method quantifies DNA residues in a sample by binding a fluorescent dye to the DNA and then using a flow cytometer or other detection equipment.
Our Process of Gene Therapy Formulation Plasmid DNA Residue Testing
DNA extraction
DNA is extracted from gene therapy formulation samples following our specific protocol.
Standard curve preparation
A series of standards of different concentrations are prepared using quantitative plasmid DNA standards by gradient dilution for the construction of standard curves.
qPCR reaction system preparation
Calculate the required reaction system components, including qPCR master mix, primer-probe mix, sample DNA, etc., according to the experimental needs.
qPCR program setting and running
Set up appropriate reaction conditions on the qPCR instrument and run the qPCR program.
Result analysis
Calculate the amount of plasmid DNA in the sample by analyzing the qPCR results, such as Ct value, standard curve slope, intercept, correlation coefficient and so on.
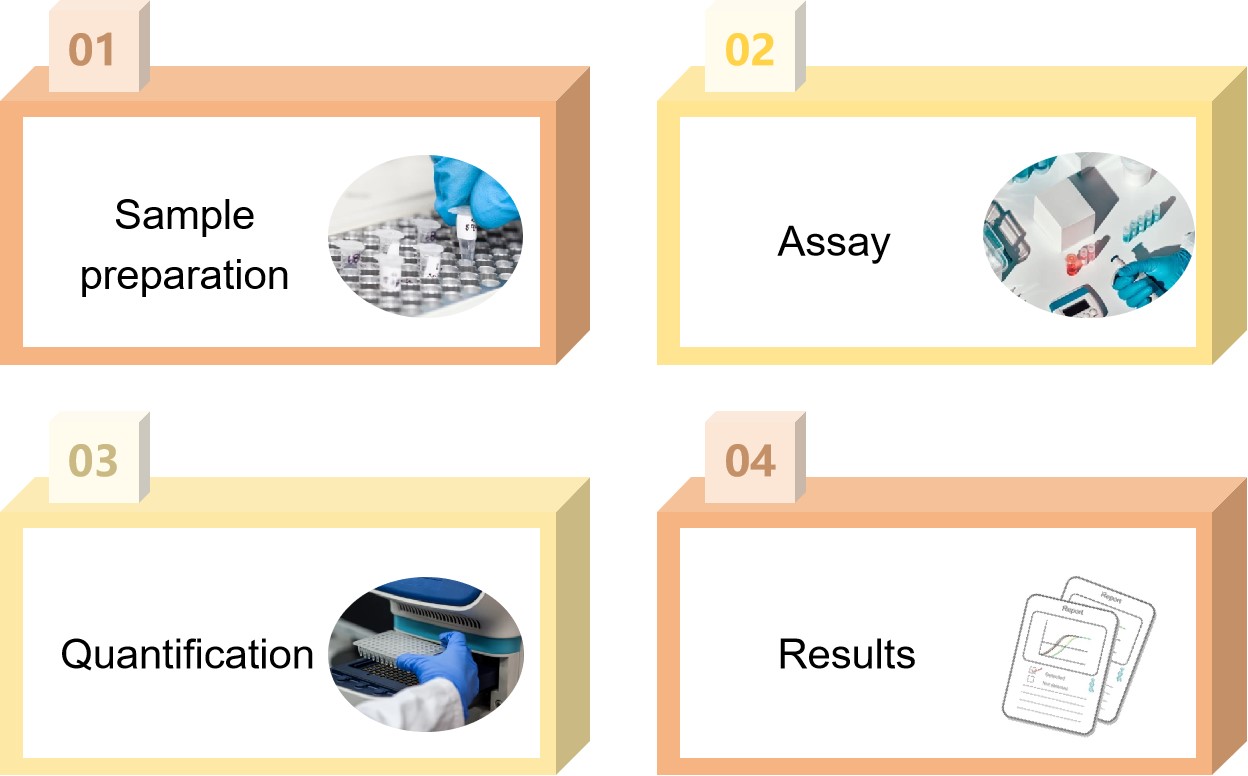 Fig.2 Our process of plasmid DNA residue testing. (CD Formulation)
Fig.2 Our process of plasmid DNA residue testing. (CD Formulation)
Our Equipment and Instruments for Plasmid DNA Residue Testing
| Equipment and Instruments |
| Real-time PCR instrument |
Fluorescent enzyme labeling instrument |
Capillary gel electrophoresis instrument |
| Automated extraction equipment |
Nucleic acid analyzer |
Ultra-clean bench |
| UV spectrophotometer |
Learn more |
Highlights of Our Gene Therapy Formulation Plasmid DNA Residue Testing
- We offer highly sensitive and specific testing methods to ensure the accuracy of test results.
- We conduct our testing in accordance with international quality management systems and regulatory agency requirements to ensure compliance with our services.
- We can provide customized solutions according to customer's project characteristics and soccer to maximize the satisfaction of different customers' needs.
- We provide one-stop service including full process support from design and build to production and quality control.
Published Data
Technology: Preparation of plasmid DNA gene carrier nanoparticles
Journal: Pharmaceuticals (Basel)
IF: 4.3
Published: 2021
The article discusses gene therapy as an alternative to chemotherapy, highlighting its ability to avoid drug resistance and reduce cellular mutations through gene carriers. The study focuses on developing nanoparticles made from a biocompatible polymer, l-tyrosine polyurethane (LTU), using specific synthesis techniques to create porous nanoparticles. These nanoparticles, containing model drugs, were evaluated for their cellular uptake and transfection efficiency in both normal and cancer cells. Various microscopy methods confirmed the nanoparticles' properties, and transfection tests showed promising results, making LTU nanoparticles suitable for gene therapy drug delivery.
CD Formulation has many years of experience in gene therapy formulation development, and we can maximize your research efforts in gene therapy formulation development. If you are interested in us, please feel free to contact us.
References
- Park SY, et al. Fabrication and Biological Activities of Plasmid DNA Gene Carrier Nanoparticles Based on Biodegradable l-Tyrosine Polyurethane. Pharmaceuticals (Basel). 2021, 15(1):17.
Related Services


 Fig.2 Our process of plasmid DNA residue testing. (CD Formulation)
Fig.2 Our process of plasmid DNA residue testing. (CD Formulation)