Gene Therapy Formulation Mycoplasma Testing
Inquiry
As gene therapy research has gotten more intense recently, monitoring and investigating the safety and dependability of biologics has taken center stage. Because Mycoplasma is a relatively common but often difficult type of contamination to remove, it is important to ensure that any biologics process involving cell culture is free of Mycoplasma contamination.
CD Formulation integrates biotechnology services and R&D, and specializes in providing comprehensive technical support and effective solutions for companies and research units related to the development of gene therapy formulations, including the quality testing of various types of cells and raw materials, and the validation of viral clearance process technology services.
The Importance of Gene Therapy Formulation Mycoplasma Testing
As a revolutionary medical treatment, it is crucial to ensure the safety of gene therapy, especially the need to ensure that therapeutic preparations are free of Mycoplasma contamination. Mycoplasma is a common microorganism that may cause contamination during cell culture, affecting cell growth and metabolism, and is difficult to remove. Therefore, testing gene therapy formulations for Mycoplasma is a critical step in ensuring their safety. Regulatory agencies have strict requirements for the safety of biologics to ensure that there is no Mycoplasma contamination during the manufacturing process. Mycoplasma testing is not only critical to ensure the safety of gene therapy, but is also a prerequisite for compliance and regulatory approval.
In conclusion, Mycoplasma testing plays an irreplaceable role in ensuring the safety, efficacy and quality control of gene therapy formulations, and is an indispensable part of the gene therapy product development and manufacturing process.

Explore Our Gene Therapy Formulation Mycoplasma Testing
Sample testing service
- Mycoplasma culture media sensitivity check (color change unit test method).
- Mycoplasma culture method.
- Mycoplasma indicator cell culture method.
- Mycoplasma qPCR method.
Sample suitability verification service
- Sample suitability verification for Mycoplasma culture method.
- Mycoplasma indicator cell culture method sample suitability validation.
Mycoplasma qPCR method methodology validation service
- Limit of detection
- Specificity
- Ruggedness
- Comparability
Our Process of Gene Therapy Formulation Mycoplasma Testing
Sample preparation
Prepare a sample of cell culture supernatant or cell suspension to be tested. If cell culture medium is used, it can be added directly into the PCR reaction solution, if it is cell suspension, it is necessary to extract DNA first.
Experimental control design
It is recommended to design experimental controls, including Positive Amplification Control (PAC), No Template Control (NTC), Negative Extraction Control (NEC), and Positive Extraction Control (PEC), in order to ensure the accuracy of the assay results.
DNA extraction
For samples requiring DNA, DNA extraction was performed.
Preparation of PCR reaction solution
Prepare PCR reaction solution and add internal quality control DNA as needed.
qPCR amplification
Put the prepared reaction solution into the fluorescence quantitative PCR instrument, set the corresponding amplification program, and carry out qPCR amplification detection.
Analysis of results
According to the qPCR results, analyze the change of fluorescence signal to determine whether there is Mycoplasma contamination in the sample.
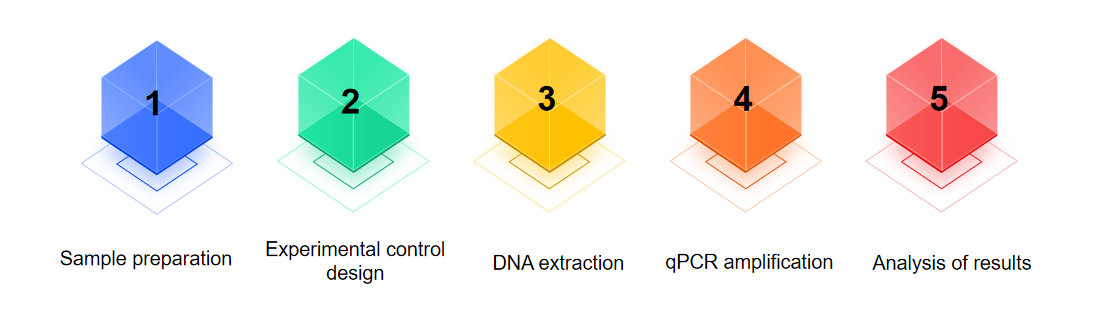 Fig.2 Our process of Mycoplasma testing. (CD Formulation)
Fig.2 Our process of Mycoplasma testing. (CD Formulation)
Our Technologies for Gene Therapy Formulation Mycoplasma Testing
| Platforms & Technologies |
Content Description |
| qPCR method |
We have Mycoplasma qPCR assay kits that meet all the requirements for Mycoplasma validation, with high assay sensitivity, specificity and durability for complete method performance validation against Mycoplasma. |
| Mycoplasma culture method |
Each medium is inoculated with a specific volume of the test material and incubated in a specific environment for 28 days to observe the changes in the medium and compare with the negative and positive control medium. |
| Indicator method |
After culturing the test material in the indicator cells, stain with specific fluorescent dyes. If the test article is contaminated with Mycoplasma, the Mycoplasma DNA attached to the cell surface can be seen under the fluorescence microscope and stained. |
Highlights of Our Gene Therapy Formulation Mycoplasma Testing
- Rapid testing. The advanced Mycoplasma detection technology we utilize provides accurate, actionable test results quickly.
- Method validation support. We provide method validation support, including specificity, detection limit and durability validation, to ensure that the assays used are scientifically reliable.
- Technical support. We provide detailed technical support and services to help customers understand the test results and provide further advice and solutions as needed.
- Flexible testing. According to the specific needs of our clients, we can provide customized testing solutions to meet the testing needs of different types and stages of gene therapy products.
Published Data
Technology: Polymerase chain reaction (PCR)
Published: 2005
Mycoplasma contamination can affect the accuracy of experimental results and the quality of the final product when developing and producing biologics. Labs should routinely test newly introduced and long-term cultivated cell lines for Mycoplasma contamination in order to guarantee the quality and safety of biological product. This article describes polymerase chain reaction (PCR) as an efficient and sensitive technique to rapidly detect the presence of Mycoplasma and identify the specific species of contamination. The entire testing process, including sample processing, DNA extraction, PCR reaction and result analysis, can be completed in less than three hours. In addition, to improve the accuracy of the test, appropriate measures need to be taken to avoid interference with the PCR reaction by inhibitors that may be present in the sample and to interpret the results correctly.
CD Formulation constantly optimizes service system and updates our technical services, aiming to provide customers with high-quality, advanced and reliable gene therapy formulations development service support. If you are interested in us, please feel free to contact us. If you are interested in us, please feel free to contact us.
References
- Uphoff CC, Drexler HG. Detection of Mycoplasma contaminations. Methods Mol Biol. 2005;290:13-23.
Related Services


 Fig.2 Our process of Mycoplasma testing. (CD Formulation)
Fig.2 Our process of Mycoplasma testing. (CD Formulation)