Gene Therapy Formulation Microbiological Testing
Inquiry
Microbiological testing is important in the quality control of gene therapy formulations, as it helps to detect contaminants that may be introduced, including exogenous viruses, bacteria, fungi, mycoplasma, and bacterial endotoxins. Microbiological testing ensures the safety and efficacy of gene therapy formulations and is critical for the subsequent application of gene therapy formulations. CD Formulation specializes in the development and testing of gene therapy formulations, and we have a comprehensive range of state-of-the-art quality control testing services to help investigators accurately test gene therapy formulations. To date, our microbiological testing has been used extensively to assess the safety and regulatory compliance of gene therapy formulations and to help developers minimize and control pathogenic bacteria, viruses and microbial toxins in their products.
The Importance of Gene Therapy Formulation Microbiological Testing
By performing accurate microbiological testing of gene therapy formulations, we not only ensure the safety and efficacy of gene therapy formulations, but also ensure quality control and compliance with regulatory agencies, thereby helping to minimize the risk of adverse health outcomes due to microbial contamination. In addition, microbiological testing enables us to comprehensively assess the effectiveness of gene therapy formulations during development and production due to different manufacturing processes, sterilization methods and other aspects.
Explore Our Gene Therapy Formulation Microbiological Testing
We provide you with rigorous microbiological testing services and the most appropriate solution for your program's characteristics to ensure that your gene therapy formulations meet microbiological testing requirements. From bioburden and sterility analysis to endotoxin testing, our suite of microbiological testing services is carefully designed to meet your unique needs.
Bioburden testing
Bioburden testing is essential to ensure the safety and performance of gene therapy formulations, as any microbial contamination may have an impact on the safety and efficacy of gene therapy formulations. We can help you optimize downstream sterility testing by regularly testing for the number of viable aerobic microorganisms.
Sterility testing
Our state-of-the-art sterility testing in isolator and cleanroom environments uses advanced inoculation and membrane filtration methods to keep your products in compliance with current requirements. To ensure validity and accuracy, we incorporate bacteriostatic testing and evaluate your samples' resistance to microbial growth. Our advanced analytical instrumentation accurately evaluates microorganisms in your product to ensure the validity of your sterility test results.
Endotoxin testing
We have a comprehensive range of bacterial endotoxin tests that are designed to meet all relevant requirements. You can choose from our qualitative gel-clot assay or our dynamic turbidimetric and colorimetric assays for quantitative accuracy. In conclusion, endotoxin testing is important to ensure the safety and efficacy of gene therapy formulations.
Our Technologies for Gene Therapy Formulation Microbiological Testing
| Platforms & Technologies |
Content Description |
| Rapid sterility testing technology |
For the special characteristics of cell and gene therapy products, such as short potency period and small batch size, rapid aseptic detection technology can accomplish microbial detection in a short period of time. |
| Realtime fluorescence quantitative PCR (qPCR) |
As a highly sensitive and accurate nucleic acid amplification technology, it can be used for rapid detection of microbial DNA with high sensitivity. |
| High-throughput sequencing technology |
The use of high-throughput sequencing technology, such as NGS technology, in the microbial detection of gene therapy formulations provides a highly efficient, accurate and informative means of detection, which can provide a comprehensive analysis of the microbial community and help improve the detection of pathogenic microorganisms. |
Highlights of Our Gene Therapy Formulation Microbiological Testing
- We are at the forefront of gene therapy formulation development and have various advanced technology platforms designed to provide accurate and efficient testing services.
- We can provide comprehensive quality control testing services for gene therapy formulations to maximize our ability to meet our customers' multifaceted testing needs.
- We have established an advanced testing technology platform to ensure efficient, rapid and accurate microbiological testing, which greatly improves the efficiency and accuracy of gene therapy formulation testing.
- We have a strict standardized system for microbiological testing of gene therapy formulations to help researchers promote the continuous development of gene therapy formulation development.
Published Data
Technology: Microbiological testing
Journal: J Clin Microbiol
IF: 5.8
Published: 2020
Sterility testing and monitoring of sterile environments for cell therapy products pose an ongoing challenge for clinical microbiology laboratories. This article describes the application of current Good Manufacturing Practices (cGMP) for sterility and mycoplasma testing based on biopharmaceutical industry standards and summarizes pharmacopoeial and rapid microbiological testing methods. Also, key regulatory requirements for laboratories performing product testing are highlighted. As the field of cellular therapy advances, laboratories need to familiarize themselves with microbiological testing regulations and cGMP practices in the biopharmaceutical industry.
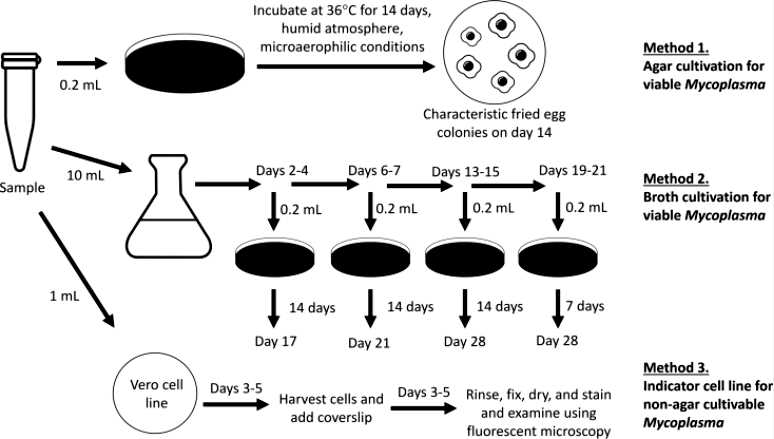 Fig.1 Mycoplasma testing methods. (Gebo JET, et al., 2020)
Fig.1 Mycoplasma testing methods. (Gebo JET, et al., 2020)
CD Formulation is committed to providing comprehensive testing services for the development of gene therapy formulations, relying on various state-of-the-art technology platforms and an experienced team of experts and technicians. If you are interested in us, please feel free to contact us.
References
- Gebo JET, et al. Sterility Testing for Cellular Therapies: What Is the Role of the Clinical Microbiology Laboratory? J Clin Microbiol. 2020, 58(7):e01492-19.
Related Services

 Fig.1 Mycoplasma testing methods. (Gebo JET, et al., 2020)
Fig.1 Mycoplasma testing methods. (Gebo JET, et al., 2020)