Gene Therapy Formulation Host Cell Residual Protein Testing
Inquiry
Host cell protein (HCP) forms a major portion of process-related impurities during the production of gene therapy formulations. The amount of residual HCP in gene therapy formulations is often considered a critical quality attribute (CQA) because it has the potential to affect product safety and efficacy. Host cells are involved in the expression of proteins related to normal cellular functions along with the expression of the target product. As a result of apoptosis, cell death, and lysis, non-essential proteins other than the target product may be released into the cell culture or fermentation supernatant. Since almost all HCPs are foreign proteins, the presence of residual HCPs in gene therapy formulations may trigger an immune response in the human body, so it is important to conduct host cell residue assays for gene therapy formulations.
CD Formulation provides comprehensive testing services for quality control of gene therapy formulations, with a particular focus on host residual cell testing. Our accurate and sensitive host protein residue assays enable researchers to ensure the safety and efficacy of their products to meet regulatory requirements.
The Importance of Gene Therapy Formulation Host Cell Residual Protein Testing
- Consider safety. HCPs, as impurities in the manufacturing process, may cause adverse reactions and affect the stability and storage of gene therapy formulations. High levels of HCPs may trigger immunogenic reactions, so testing is needed to control their levels.
- Meeting regulatory requirements. Testing gene therapy formulations for HCPs helps to meet the stringent requirements of the relevant regulatory agencies to control HCP levels to ensure the safety and quality of biologics.
- Controlling residual proteins. HCP residues are a critical step in the quality control of gene therapy formulations, and testing for HCPs ensure that host cell proteins in gene therapy formulations are kept at an acceptably low level.
- Developing relevant strategies. HCPs testing helps to develop and implement effective removal strategies to minimize HCPs in final gene therapy formulations.
Explore Our Gene Therapy Formulation Host Cell Residual Protein Testing
When selecting a host cell protein residue testing method, we consider various factors in conjunction with the characteristics and requirements of our clients' programs, including the required sensitivity and accuracy, and the complexity of the samples. Often, we combine multiple methods to ensure the quality and safety of gene therapy agents. Below are a few of some of the techniques we utilize most often.
Enzyme-linked immunosorbent assay (ELISA)
This technique utilizes specific antibodies to bind to host proteins and quantifies the residual levels of host proteins by measuring the antigen-antibody reaction. It has the advantages of high sensitivity, high throughput and ease of use in quantifying total HCP concentration.
Mass spectrometry (MS)
We use mass spectrometry to provide qualitative and quantitative information on HCPs by ionizing sample molecules and separating and detecting them based on their mass-to-charge ratio.
Two-dimensional polyacrylamide gel electrophoresis-mass spectrometry method (2D-PAGE-MS)
This technique separates proteins based on their molecular weight and isoelectric point differences, and protein identification is performed by mass spectrometry. This technique detects a wider range of HCPs and provides a comprehensive protein profile.
Bioactivity assay
Validation of residues by determining the effect of host protein on biological activity, providing information on the direct effect of host protein residues on the activity of gene therapy formulations.
Host Protein Residue Testing Classification
- Sample source. We can accept samples from different sources. For example, we can use process samples and end-products from E. coli fermentation and purified plasmid production process, or process samples and end-products from specific cell fermentation and purified lentivirus production process.
- Test method. Elisa method, our kits are quality controlled to meet the regulatory requirements of the relevant authorities.
- Quantification range. The quantitative range is different according to different sample sources, for example, 1-100ng/ml for E. coli source and 2-200ng/ml for cellular fermentation source.
- QC requirements. Standard curve R2>0.99, spiked recovery is 80-120%.
Our Technologies for Gene Therapy Formulation Host Cell Residual Protein Testing
| Platforms & Technologies |
Content Description |
| Microprotein residue detection |
Detection of microprotein residues in gene therapy preparations by advanced technologies such as high performance liquid chromatography and mass spectrometry. |
| Host protein residue detection |
Host protein is one of the impurities that may be generated during the production of gene therapy preparations. Detection of host protein residues helps to assess the safety and purity of the product. Common host protein residue detection methods include immunoassay, mass spectrometry, etc. |
| Mycobacterial protein residue detection |
Detection of mycobacterial protein residues helps to assess the purity and safety of gene therapy preparations. Commonly used detection methods include enzyme-linked immunosorbent assay (ELISA) and high performance liquid chromatography. |
Highlights of Our Gene Therapy Formulation Host Cell Residual Protein Testing
- We have professional testing personnel who can master various testing methods and techniques to ensure the accuracy and reliability of the test results.
- We have good service quality and perfect after-sales support, which can ensure that our customers get timely and professional help and support in the testing process.
- We have the relevant qualifications and certifications to ensure the accuracy and reliability of the test results.
- We have advanced testing equipment, which can improve the sensitivity and accuracy of testing.
Published Data
Technology: Host cell protein analysis
Journal: Anal Bioanal Chem
IF: 4.3
Published: 2022
Manufacturers of biologics must ensure drug purity and patient safety by removing host cell impurities, such as host cell proteins (HCPs), which can affect product quality and efficacy. The standard technique for HCP analysis is HCP-ELISA, valued for its simplicity, quick analysis, and high sensitivity. However, liquid chromatography mass spectrometry (LC-MS) is gaining popularity for its ability to identify and quantify individual HCPs, addressing some limitations of ELISA. This article explores advances in HCP analysis, highlighting case studies and new strategies using both ELISA and LC-MS to improve HCP control during biologics development.
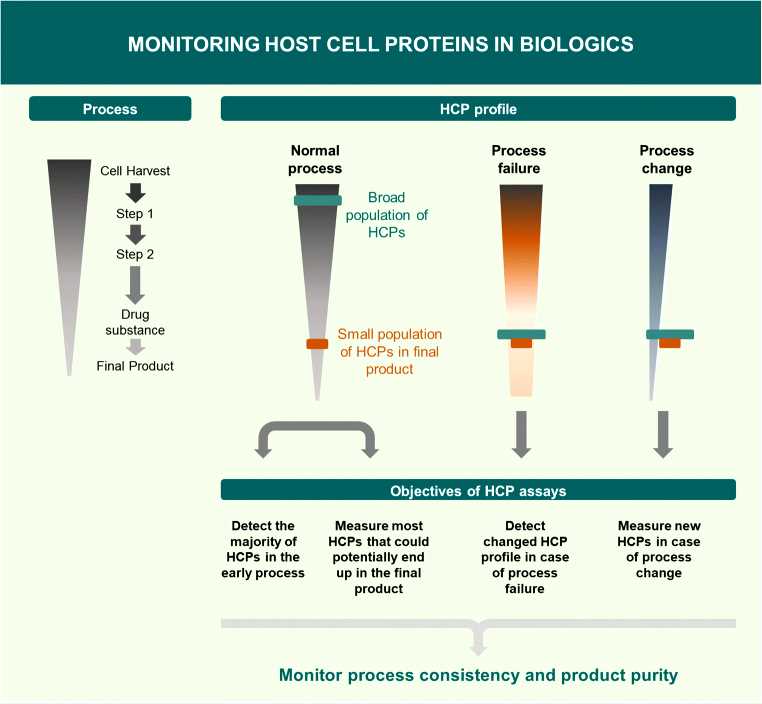 Fig.1 Objectives of host cell protein assays in biologics. (Pilely K, et al., 2022)
Fig.1 Objectives of host cell protein assays in biologics. (Pilely K, et al., 2022)
CD Formulation has state-of-the-art analytical equipment and methods to support the development of gene therapy formulations with comprehensive HCP testing services. If you are interested in us, please feel free to contact us.
References
- Pilely K, et al. Monitoring process-related impurities in biologics-host cell protein analysis. Anal Bioanal Chem. 2022, 414(2):747-758.
Related Services

 Fig.1 Objectives of host cell protein assays in biologics. (Pilely K, et al., 2022)
Fig.1 Objectives of host cell protein assays in biologics. (Pilely K, et al., 2022)