Gene Therapy Formulation Exogenous Virus Testing
Inquiry
Detection of exogenous viruses is a critical quality control step in the development of gene therapy formulations. Exogenous viruses may originate from various sources, including raw materials used in the production process, cellular matrix, operating environment, etc. Therefore, ensuring the safety and efficacy of gene therapy products requires strict detection and control of exogenous viruses. Therefore, to ensure the safety and efficacy of gene therapy products, stringent testing and control of exogenous viruses is required. CD Formulation provides comprehensive technical support in the development of gene therapy formulations, especially for quality control including exogenous virus testing. Our testing process includes exogenous virus screening, exogenous virus identification and virus removal to minimize the impact of viruses on gene therapy formulations to ensure that the target product meets quality standards and regulatory requirements.
The Importance of Gene Therapy Formulation Exogenous Virus Testing
Exogenous virus testing is essential to ensure the safety, efficacy and quality control of gene therapy products and is an indispensable part of the gene therapy field. The importance of exogenous virus testing will be further emphasized with the development of technology and the improvement of regulatory requirements.
- Ensure product safety. Gene therapy products that are contaminated with exogenous viruses may pose a health threat to patients. Therefore, testing for exogenous viruses is a basic requirement for ensuring patient safety and a prerequisite for guaranteeing the safety of gene therapy formulations.
- Compliance with regulatory requirements. Regulatory agencies around the world require rigorous testing of biologics for exogenous viruses to ensure product safety. Compliance with these regulations for exogenous virus testing is critical for the subsequent approval and promotion of gene therapy formulations.
- Maintaining product quality. Contamination with exogenous viruses may affect the quality of gene therapy products, and by detecting and controlling exogenous virus contamination, product consistency and reliability can be assured.
Explore Our Gene Therapy Formulation Exogenous Virus Testing
Real-time PCR (qPCR) assay
- We use qPCR assay to detect the presence of infected exogenous viruses in gene therapy formulations.
- We can target different sources of viral factors and detect DNA residue levels with high sensitivity, high throughput, high efficiency, and high accuracy through our specialized sample pre-treatment and qPCR assays.
Cell culture method
We apply cell platform or let culture method in exogenous virus detection, and strictly follow the corresponding norms and standard operation procedures to ensure the accuracy and reliability of the test results, our main process is shown below.
- Determine the appropriate cell line.
- Preparation of samples.
- Add sample.
- Observe and record.
- Analyze and confirm.
Due to the complex preparation process and lyophilization, we also need to optimize and validate different biologics according to their characteristics.
Digital PCR (dPCR)
- Exogenous viruses can be detected using dPCR. Based on the characteristics of dPCR, it can make up for the shortcomings of qPCR technology, as follows.
- The ability to directly count the copy number of the target sequence without relying on a reference standard curve.
- Meager copy numbers can be detected.
- More tolerant to inhibitory substances in the sample.
Next-generation sequencing (NGS)
Adopting NGS technology has the advantages of high sensitivity, high specificity, multiplex detection, no need for a priori knowledge, and high throughput. However, there are many potential problems, such as sequencing errors, contamination. We can misinterpret, etc., so it is necessary to follow a strict standardized procedure to ensure the accuracy and reliability of the test results. The main processes we use when utilizing sequencing methods for exogenous virus detection are shown below.
- Establishment of a library for virus detection.
- Sequencing and data analysis.
- Verification and validation.
Our Technologies for Gene Therapy Formulation Exogenous Virus Testing
| Platforms & Technologies |
Content Description |
| Cell culture technology |
We have co-infected cells by co-infecting samples with helper viruses, then passaging the transduced cell lysates for serial amplification, and finally using qPCR to detect specific genes. |
| Quantitative PCR (qPCR) technology |
This is a rapid and sensitive assay that can be used to detect DNA or RNA of specific viruses. qPCR is commonly used to detect rcAAV contamination during viral vector production and for quantitative analysis of viral vectors. |
| Bioinformatics analysis technology |
We combine high-throughput sequencing data with bioinformatics tools that can be used to analyze and identify viral sequences, which is particularly important for the detection of unknown viruses. |
| Viral clearance validation technology |
We need to validate the effectiveness of the viral clearance step in the manufacturing process of our gene therapy products to ensure the safety of the products and to demonstrate that the viral clearance step in the manufacturing process is capable of removing or inactivating the viruses. |
Highlights of Our Gene Therapy Formulation Exogenous Virus Testing
- We offer a comprehensive range of exogenous virus testing services with an experienced team of expert technicians and state-of-the-art laboratory equipment.
- We offer comprehensive performance validation services for customized assays and sample testing using a wide range of state-of-the-art methodologies.
- We provide GMP-compliant NGS solutions for exogenous and specific virus detection, viral vector and plasmid identification services.
- We provide end-to-end services including research packaging, process development, analytical method development, cGMP manufacturing, and testing services.
- We can provide one-stop gene therapy formulation development and testing solutions according to customers' requirements, to maximize the satisfaction of customers' all-round needs.
Published Data
Technology: Real-time PCR (qPCR)
Journal: Mol Ther Methods Clin Dev
IF: 4.6
Published: 2023
Gene therapy technology has made remarkable progress in treating genetic diseases and can now tackle thousands of diseases. During drug development, the establishment of analytical methods is crucial for clinical trials and commercial production. Genetic testing technologies play a key role in ensuring the safety, efficacy, and stability of drug components. Although nucleic acid-based assays are quite mature, there are unique biological, technological, and regulatory challenges to be considered in gene therapy. This article describes the application of nucleic acid-based gene therapy testing for method development and validation, with a particular focus on adeno-associated virus (AAV) vectors and their cotransfection factors. Comparing quantitative PCR and droplet digitization techniques explores how technological improvements can be made while following regulatory guidance. Specifics of analytical method development are demonstrated through examples of validation parameters for AAV genomic titers. In addition, this article suggests several possible directions for improvement, such as next-generation sequencing and artificial intelligence, to facilitate future innovations in analytical testing.
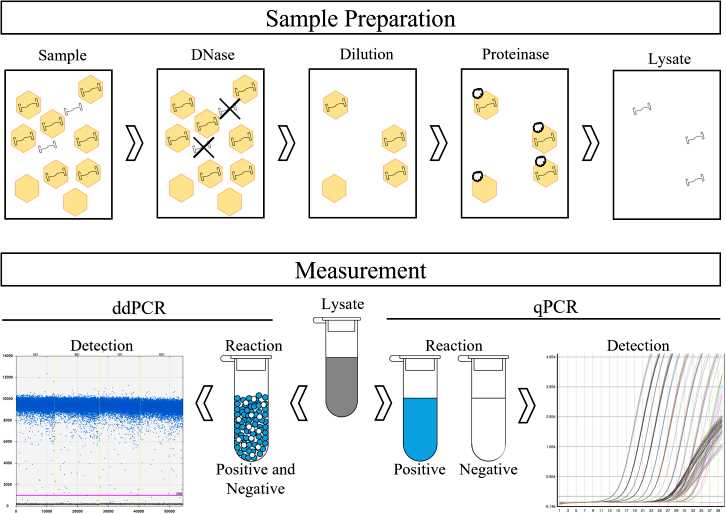 Fig.2 Example of adeno-associated virus gene therapy sample preparation before PCR analysis. (Blay E, et al., 2023)
Fig.2 Example of adeno-associated virus gene therapy sample preparation before PCR analysis. (Blay E, et al., 2023)
CD Formulation can utilize qPCR, cell culture method, dPCR, NGS and other methods to detect exogenous viruses in the developed gene therapy formulations and issue appropriate test reports. We ensure the reliability and accuracy of our tests and look forward to working with you in good faith. If you are interested in us, please feel free to contact us.
References
- Blay E, et al. PCR-based analytics of gene therapies using adeno-associated virus vectors: Considerations for cGMP method development. Mol Ther Methods Clin Dev. 2023, 31:101132.
Related Services


 Fig.2 Example of adeno-associated virus gene therapy sample preparation before PCR analysis. (Blay E, et al., 2023)
Fig.2 Example of adeno-associated virus gene therapy sample preparation before PCR analysis. (Blay E, et al., 2023)