Gene Therapy Formulation Accelerated Stability Testing
Inquiry
Accelerated stability testing of gene therapy formulations is a test performed during the development of gene therapy formulations to assess the stability of gene therapy formulations during storage and use. CD Formulation provides effective technical support and comprehensive testing solutions for accelerated stability testing of gene therapy formulations, which are often performed under more severe conditions than the actual storage conditions. The tests we offer are typically performed under more severe conditions than actual storage conditions, such as elevated temperatures to accelerate the degradation process of the formulation, to quickly obtain data on the stability of the gene therapy formulation. This service helps researchers predict the expiration date and stability of a product under normal storage conditions and provides a basis for determining the correct storage conditions and packaging.
The Importance of Gene Therapy Formulation Accelerated Stability Testing
Accelerated stability testing helps to quickly predict the long-term stability of gene therapy formulations under routine storage conditions. By testing the rate of degradation of a formulation under stressful conditions such as elevated temperature and humidity, it is possible to assess how it may deteriorate in a normal storage environment. This is critical for optimizing product formulations, selecting appropriate storage conditions, and determining product expiration dates. Specifically, this is mainly reflected in the following aspects.
- Predicting product expiration dates. By simulating the conditions that a product may encounter during storage, accelerated stability testing can help predict a product's expiration date under normal storage conditions.
- Evaluating storage conditions. This testing helps determine the most appropriate storage conditions for gene therapy products to ensure their stability and effectiveness.
- Guide formulation prescription and packaging material selection. The test results can be used to determine the reasonableness of formulation prescriptions and packaging materials, and provide a basis for the product manufacturing process.
- Regulatory compliance. Meet the requirements of the relevant regulatory agencies for the stability study of biologics, laying the foundation for the subsequent commercialization of the product.
Explore Our Gene Therapy Formulation Accelerated Stability Testing
Test design and program customization
First of all, we can help our clients to develop the most suitable accelerated stability test program according to the product characteristics, including the determination of the test conditions, such as temperature, humidity, and the evaluation time point and so on. In this process, we need to take into account the complex degradation pathways of gene therapy formulations, and consider various of factors to provide customers with the most scientific and reasonable measurement program.
Sample preparation and storage
We provide professional sample preparation and storage services to ensure that all samples are handled under standardized conditions to avoid interference with test results by external factors. We simulate the degradation that may occur during prolonged storage by storing target samples under accelerated conditions.
Degradation analysis and data modeling
We use advanced analytical techniques, such as HPLC and LC-MS, to monitor the degradation of gene therapy formulations at different time points. Based on the degradation data, we perform modeling of acceleration factors and degradation rates to predict stability under routine storage conditions.
Reporting and conclusion
At the end of the test, combined with the analysis results, we will provide a detailed analysis report covering key data such as degradation rate, and predicted stability duration, and give suggestions on storage conditions, formulation optimization, etc., to help customers make scientific development decisions when developing gene therapy formulations.
Our Process of Gene Therapy Formulation Accelerated Stability Testing
- Sample preparation. Selection of representative batch samples for testing.
- Condition setting. Tests are carried out in more challenging conditions, usually with variables like light, humidity, and temperature.
- Time point setting. Determining the time point for sampling.
- Test items. Perform testing on gene therapy agents including, but not limited to, physical, chemical, and biological properties.
- Data analysis. The data collected is analyzed to determine the stability trends of the formulation.
- Stability prediction. Make use of the obtained target formulation's stability and expiration date while storing it normally.
- Outcome assessment. Evaluate the stability of the gene therapy formulation and evaluate whether any modifications to the formulation or storage conditions are required based on the data analysis results.
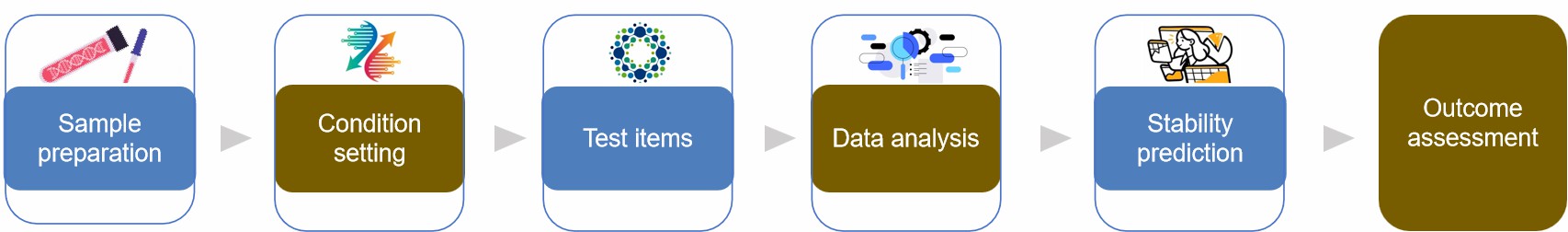 Fig.1 Gene therapy formulation accelerated stability testing process. (CD Formulation)
Fig.1 Gene therapy formulation accelerated stability testing process. (CD Formulation)
Our Technologies for Gene Therapy Formulation Accelerated Stability Testing
| Platforms & Technologies |
Content Description |
| Plasmid vector analysis |
Using advanced molecular biology techniques to analyze the stability of plasmid vectors, with plasmid vector stability assays we can ensure the stability of plasmid vectors in gene therapy formulations. |
| Viral vector analysis |
Includes adeno-associated virus vector (AAV) analysis, using various advanced techniques to detect solid and empty capsid rates. |
| Genomic titer assays |
Genomic titer assays play an important role in the accelerated stability testing of gene therapy formulations, and we commonly use qPCR and ddPCR to test the titer of viral vector-based products. |
| rcAAV residue detection |
Detection using AAV samples infected with sensitive cells combined with qPCR analysis. |
| Whole genome sequencing |
Uses next-generation sequencing technology to provide comprehensive genetic stability test results. |
| Bioinformatics analysis |
Data analysis using bioinformatics tools in conjunction with whole genome sequencing results. |
Highlights of Our Gene Therapy Formulation Accelerated Stability Testing
- Professional team. Our experts and technical team have rich experience in gene therapy formulation development and stability studies and can provide customized accelerated stability assay services.
- Advanced equipment and technology. We are equipped with the world's leading analytical and testing equipment, which can provide accurate and reliable degradation analysis data and ensure the efficiency and accuracy of the results.
- Comprehensive assay services. We provide one-stop service from stability test design to final data reporting, ensuring that gene therapy formulations remain efficient and smooth during the development process.
- Customized solutions. We provide customized stability testing solutions based on the characteristics of each gene therapy formulation and the needs of our clients.
Published Data
Technology: Accelerated stability testing
Journal: AAPS PharmSciTech
IF: 3.3
Published: 2021
In pharmaceutical development, long-term stability testing is essential to ensure product stability, as issues discovered later can lead to costly re-formulations and delays. Predicting stability early in development is challenging, but the Accelerated Stability Assessment Program (ASAP), which uses a modified Arrhenius equation and isoconversion method, offers a quicker alternative for evaluating product stability and shelf-life. Although most studies focus on small drug molecules, this study applied ASAP to ascorbic acid (AA) and a cyclic hexapeptide (cFEE). The results, based on degradation products, showed that ASAP accurately predicted stability, validating its usefulness for long-term stability assessment.
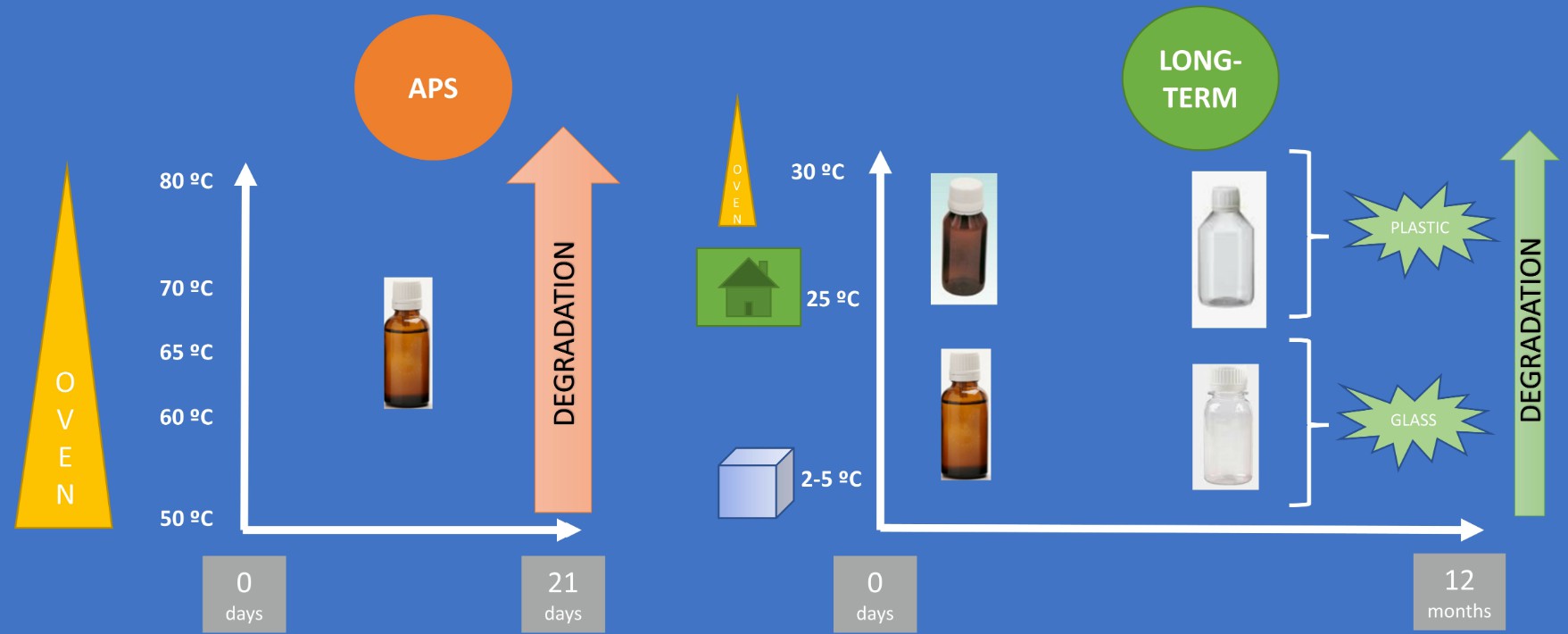 Fig.2 Application of accelerated predictive stability studies to formulations for shelf-life assessment. (Legrand P, et al. 2021)
Fig.2 Application of accelerated predictive stability studies to formulations for shelf-life assessment. (Legrand P, et al. 2021)
Accelerated stability testing is an indispensable part of the gene therapy formulation development process, which plays a crucial role in ensuring the safety, efficacy and quality control of the product. CD Formulation, as a professional gene therapy formulation development company, aims to provide comprehensive solutions for gene therapy formulation development. If you are interested in us, please feel free to contact us.
References
- Legrand P, et al. Accelerated Stability Assessment Program to Predict Long-term Stability of Drugs: Application to Ascorbic Acid and to a Cyclic Hexapeptide. AAPS PharmSciTech. 2021, 22(7):234.
Related Services

 Fig.1 Gene therapy formulation accelerated stability testing process. (CD Formulation)
Fig.1 Gene therapy formulation accelerated stability testing process. (CD Formulation) Fig.2 Application of accelerated predictive stability studies to formulations for shelf-life assessment. (Legrand P, et al. 2021)
Fig.2 Application of accelerated predictive stability studies to formulations for shelf-life assessment. (Legrand P, et al. 2021)