VLP-Based Nucleic Acid Delivery System Development
InquiryCD Formulation is a leading company in viral vector and virus-like particle (VLP) engineering and dedicated to developing advanced nucleic acid drug delivery platforms. With over a decade of experience designing diverse VLP systems, our interdisciplinary team has extensive experience rationally engineering the structure and function of VLP capsids derived from viruses such as HIV, HBV, and HCV.
How are VLPs Used for Nucleic Acid Delivery?
VLPs show great potential as delivery vectors for nucleic acid therapeutics. VLPs are non-infectious protein cages that self-assemble into structures that resemble native viruses. They can be engineered to densely package and protect nucleic acids like DNA and RNA from degradation while evading host immune detection. VLPs also actively deliver their cargo to specific cell types through interactions with surface receptors, making them a promising candidate for targeted nucleic acid delivery through various routes.
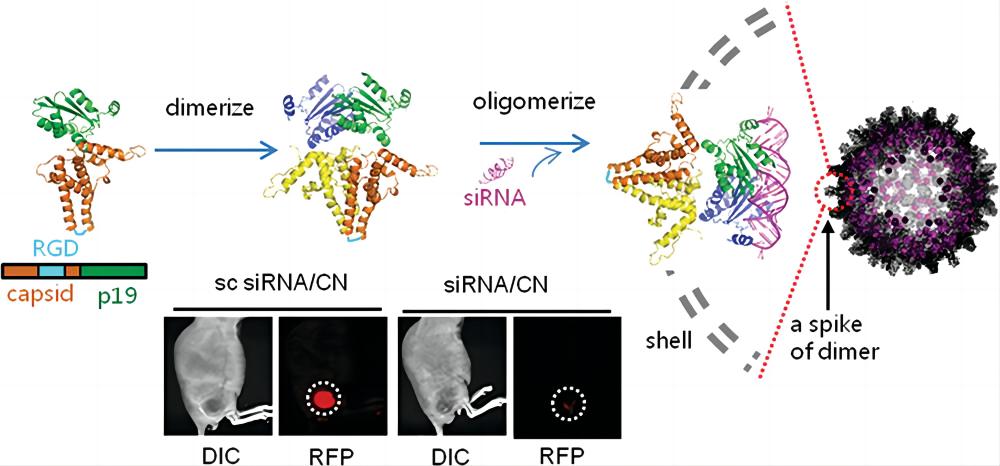 Self-assembly process of VLPs for siRNA delivery (Choi K.; et al. 2013)
Self-assembly process of VLPs for siRNA delivery (Choi K.; et al. 2013)
Types of VLPs We Can Develop
Leveraging our expertise in virus capsid engineering, we produce non-enveloped virus-like particles by genetically modifying and expressing capsid proteins in insect or mammalian cells. These self-assembled VLPs are optimized through rational design to densely encapsulate DNA, RNA or gene editing payloads for targeted cell or tissue transfection applications.
We create enveloped virus-like particles by co-expressing modified viral structural and envelope proteins. During assembly and budding, nucleic acids are packaged inside capsid cores surrounded by engineered lipid envelopes. Surface modifications facilitate tissue-specific interactions and cell binding for higher transfection rates upon delivery.
Our Expression Platforms for VLP Development
 Bacteria
Bacteria
 Yeasts
Yeasts
 Animal/plant cells
Animal/plant cells
Our VLP-Based Nucleic Acid Delivery System Development Process
| Steps |
Contents |
Turnaround time |
| Target Identification |
- Based on customer needs, we analyze viral structure databases and literature to select capsids with optimal size (20-100nm), symmetry, and stability.
|
1-2 weeks |
| Capsid Engineering |
- Molecular structures guide rational mutagenesis of structural genes to introduce peptide motifs enhancing nucleic acid packaging density within the capsid interior while maintaining particle integrity.
|
1-2 weeks |
| Expression Systems Selection |
- Viral capsid genes are expressed using scalable adenovirus, baculovirus-infected insect cells, HEK293 or E. coli systems optimized for production yield, fidelity and purity.
|
2-3 weeks |
| Characterization |
- VLP morphology, monodispersity and antigenicity are assessed using cryo-EM, DLS, and western blot.
- Encapsulation efficiency and stability are quantified using analytical ultracentrifugation, RNase protection and thermal denaturation assays.
|
1-3 working days |
| Surface Modification |
- Cell-targeting ligands like antibodies, peptides are genetically appended or chemically conjugated to VLPs based on binding affinity analysis and spatial orientation considerations from homology modeling.
|
2-3 weeks |
| In Vitro Testing |
- VLPs are incubated with cell lines to quantitatively track the import kinetics, expression duration and transduction efficiency of nucleic acid via live-cell fluorescence microscopy, flow cytometry and sequencing-based readouts.
|
2-5 weeks |
| Results Delivery |
- Virus-like particle samples (if available).
- Project reports and product descriptions.
|
Depends on the specific project |
Advantages of Our VLP-Based Nucleic Acid Delivery System Development Services
- Utilizing VLPs as delivery vehicles offers inherent safety advantages compared to viral vectors, as VLPs lack viral genetic material, reducing the risk of viral replication or integration into the host genome.
- We specialize in developing VLPs based on various platforms, including non-enveloped and enveloped VLPs, retrovirus-based VLPs, and plant-derived VLPs, providing flexibility to meet diverse research and therapeutic needs.
- Our expertise allows for precise engineering and modification of VLPs to ensure efficient encapsulation and protection of nucleic acids.
- By incorporating surface modifications and targeting ligands, our VLPs can be tailored to specific cell types or tissues.
At CD Formulation, our experts excel at precise engineering and customization of VLPs, ensuring efficient encapsulation and protection of nucleic acids for optimal delivery. Contact us today to discuss your specific needs and leverage the advantages of our VLP technology to accelerate the development of nucleic acid therapeutics!
References
- Zhang P.; et al. Increased neutralization potency and breadth elicited by a SARS-CoV-2 mRNA vaccine forming virus-like particles. Proc Natl Acad Sci, 2023, 120(29): e2305896120.
- Choi K.; et al. Systemic delivery of siRNA by chimeric capsid protein: tumor targeting and RNAi activity in vivo. Mol Pharm. 2013, 10(1):18–25.
- Zdanowicz M.; Chroboczek J. Virus-like particles as drug delivery vectors. Acta Biochim Pol. 2016, 63(3):469-73.
Related Services



 Self-assembly process of VLPs for siRNA delivery (Choi K.; et al. 2013)
Self-assembly process of VLPs for siRNA delivery (Choi K.; et al. 2013) Bacteria
Bacteria Yeasts
Yeasts Animal/plant cells
Animal/plant cells