Viral Vector Development for Nucleic Acid Delivery
InquiryWith years of experience, CD Formulation provides end-to-end services for the development of customized viral vectors aimed at delivering nucleic acid-based therapeutics. Our services include rational vector design through genome editing, cell/tissue targeting modifications, molecular cloning, multigene vector construction and analytical characterization.
Viral Vectors for Nucleic Acid Delivery
Viral vectors are powerful tools utilized for nucleic acid delivery in various biomedical applications. These engineered viruses, derived from naturally occurring viruses, have been modified to remove their pathogenic properties while retaining their ability to efficiently deliver genetic material to target cells. Viral vectors can transfer nucleic acids such as DNA, RNA, or gene-editing tools, enabling precise manipulation of cellular functions or expression of therapeutic genes. Viral vectors have emerged as valuable tools in gene therapy, vaccine development, and biomedical research, offering immense potential for advancing precision medicine and treating genetic disorders.
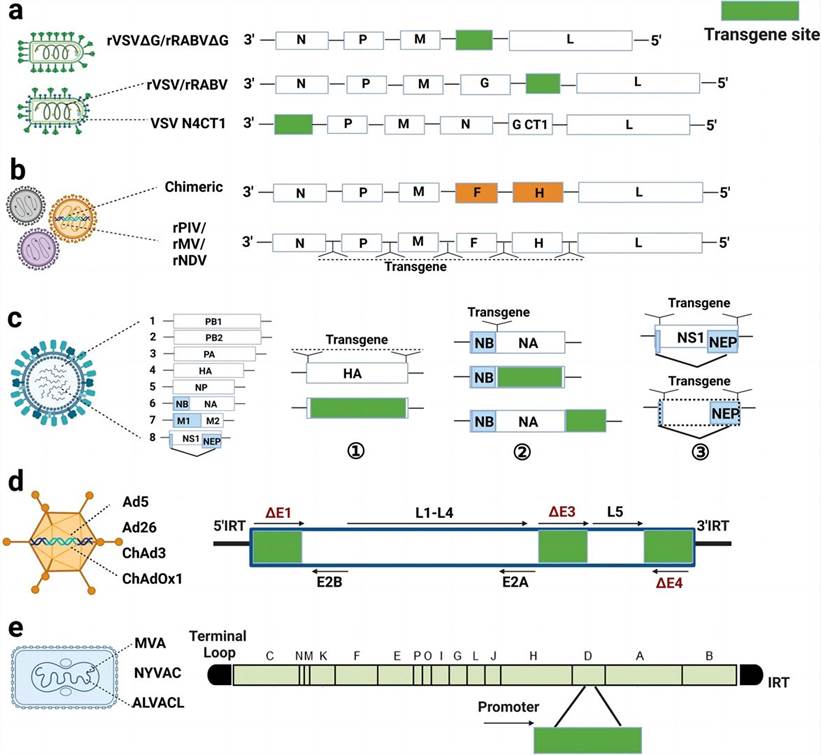 Viral vector types and design strategies (Wang S.; et al. 2023)
Viral vector types and design strategies (Wang S.; et al. 2023)
What Can We Do to Develop Viral Vectors for Nucleic Acid Delivery?
| Viral Vector Selection |
- Different viruses, such as adenoviruses (AdVs), poxviruses, lentiviruses, or influenza viruses (IFVs), offer unique characteristics and advantages for nucleic acid delivery.
- Factors such as viral tropism, genetic stability, immunogenicity, and safety profiles must be considered in selecting the most suitable viral vector for the desired application.
|
| Cell Line Selection |
- Stable cell lines for reliable data recording.
- Serum-free adherent cells or suspension cells are available for viral vector production.
- Transient or stable expression systems.
|
| Genetic Engineering |
- We use genetic engineering techniques to modify viral genomes.
- The desired nucleic acid sequence (usually a therapeutic gene or antigen) will be inserted into the viral genome.
|
| Vector Optimization |
- We fine-tune viral vector genes to balance efficient replication, high-level gene expression, and host immune responses.
- Strategies such as codon optimization, inclusion of regulatory elements, and selection of appropriate viral promoters are used to optimize vector performance.
|
| Quality Control |
- Viral vector capsid protein purity detection.
- Viral vector genome size and purity detection.
- Viral vector biological activity detection.
|
Types of Viral Vectors We Can Develop for Nucleic Acid Delivery
| NNSVs |
- The viral glycoprotein gene is deleted and replaced with the gene of interest (NNSVΔG or NNSVΔF).
- The carrier glycoprotein gene (rNSSV) is retained while additional transcription units are incorporated.
- Representative viral vectors: VSV, RABV, PIV, MeV, NDV.
|
| IFVs |
- Foreign genes are inserted into specific regions of viral segments (e.g., HA receptor binding sites) while retaining essential viral function and replication capacity.
|
| AdVs |
- Replication-competent or replication-deficient vectors are created by manipulating specific genes, such as E1 and E3, allowing efficient delivery of desired genes to target cells.
|
| Poxviruses |
- Taking advantage of the genetic stability and attenuation properties of the virus, the desired gene can be inserted while maintaining immunogenicity.
|
Nonsegmented negative‐strand RNA viruses (NNSVs); Segmented RNA (IFVs); Adenoviruses (AdVs); Vesicular stomatitis virus (VSV); Rabies virus (RABV); Parainfluenza virus (PIV); Measles virus (MeV); Newcastle disease virus (NDV)
Applications of Our Viral Vector Development Services for Nucleic Acid Delivery
 Nucleic Acid Vaccine Development
Nucleic Acid Vaccine Development
 Gene Therapy Development
Gene Therapy Development
Advantages of Our Viral Vector Development Services for Nucleic Acid Delivery
- We are committed to developing viral vectors with high titer, high purity, low endotoxin and high in vivo infection efficiency.
- We provide customized solutions and professional technical guidance for viral vector construction such as overexpression, interference, and CRISPR technology.
- We have a strong artificial intelligence and bioinformatics team to provide viral vector development and optimization services.
- We can identify the most suitable viral vector system for your specific application, ensuring compatibility and efficacy.
- By selectively disabling or deleting specific viral genes necessary for replication in target cells, we can ensure a safe and controlled delivery system for nucleic acids.
CD Formulation provides comprehensive services for the engineering and optimization of viral vectors to deliver nucleic acid-based therapeutics, including gene vaccines and gene therapies. If your organization is pursuing nucleic acid therapeutics utilizing viral vectors, please contact us to discuss how we can help design and qualify the optimal vector system for your research program.
References
- Wang S.; et al. Viral vectored vaccines: design, development, preventive and therapeutic applications in human diseases. Signal Transduct Target Ther. 2023, 8(1):149.
- Zhao Z.M.; et al. Viral vector-based gene therapies in the clinic. Bioeng Transl Med. 2021, 20;7(1):e10258.
- Bulcha J.T.; et al. Viral vector platforms within the gene therapy landscape. Signal Transduct Target Ther. 2021, 6(1):53.
Related Services



 Viral vector types and design strategies (Wang S.; et al. 2023)
Viral vector types and design strategies (Wang S.; et al. 2023) Nucleic Acid Vaccine Development
Nucleic Acid Vaccine Development Gene Therapy Development
Gene Therapy Development