CD Formulation can provide one-stop services for stability testing includinginfluencing factor tests, accelerated tests and long-term tests, etc.
Why to Perform Stability Testing?

Stability testing is an important part of the entire biological product development, clinical, marketing and post-marketing quality research. It is the basis for formulating the product validity period and provides the basis for the production process, preparation prescription, packaging materials, storage, transportation conditions, etc. of the drug. It is also the basis for formulating product quality standards. Biological products are more sensitive to environmental factors such as temperature, humidity, and light. In order to ensure their safety and effectiveness and avoid inactivation or degradation, corresponding stability tests must be carried out based on the characteristics of the product.
Our Stability Testing Services
Stability testing of biological products generally includes long-term stability testing, accelerated stability testing, and mandatory testing (also called influencing factor testing, which is an exploration of stability testing). CD Formulation will develop personalized proposals to perform stability testing for bio-pharmaceuticals based on the customer's needs. We can provide a series of stability testing services for global researchers, including but not limited to:
- Influencing Factor Testing
Influencing factor testing is generally conducted in harsher and more extreme environments. Its purpose is to evaluate the degradation pathway and degree of damage of the product. It is also used to evaluate the effectiveness of product impurity (degradation product) analysis methods.
Accelerated testing is generally conducted between long-term and mandatory testing conditions, and its purpose is to evaluate the impact of short-term deviations in conditions during transportation and storage on the product (including biodegradation or physical changes, etc.).
Long-term stability tests are generally conducted under recommended storage conditions, which can be used as an important basis for formulating product storage conditions and validity periods.
Our Testing Items for Stability
The test items of the stability test should cover those contents that are easy to change during storage and may affect product quality (safety, effectiveness). The bio-pharmaceutical stability testing items we provide include but are not limited to the following items.
- Physical and chemical tests: appearance (color and clarity), visible foreign matter and insoluble particles after solution, powder or freeze-dried powder is dissolved, pH value, osmotic pressure, moisture content of powders and freeze-dried preparations, loading volume of liquid dosage forms, etc.
- Identification: Structural confirmation of the final product.
- Content: Protein content, additives (such as stabilizers, preservatives) or excipient content, etc.
- Biological activity: Potency.
- Degradation products: Consider not only the degradation of the product but also the degradation of additives such as stabilizers, preservatives or excipients.
- Microbiology: Sterility, mycoplasma, etc.
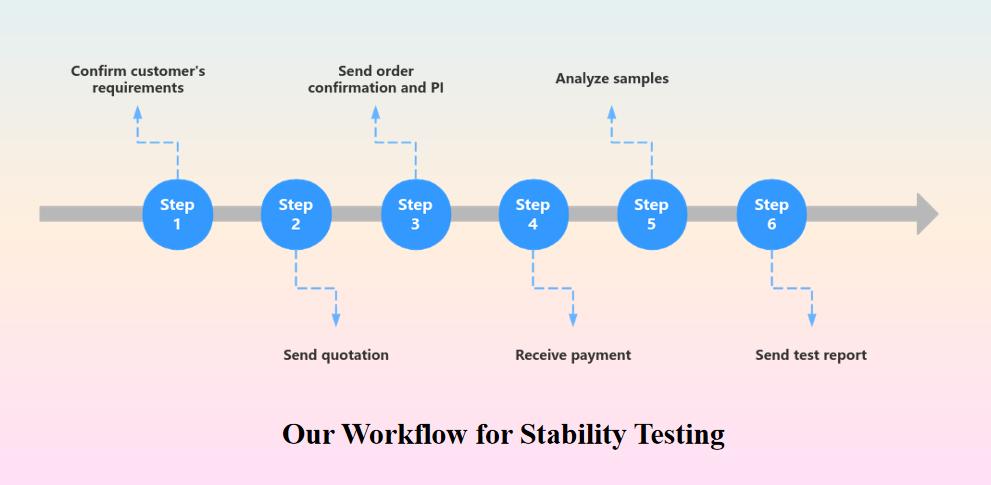
Our Workflow for Stability Testing
Here are following six steps to easily solve your requirements.
Advantages of Our Services
- We can provide you with the professional stability testing proposal, including testing condition, agents and equipment, sampling time points, testing items, testing record, and testing conclusion, etc.
- Once the order is confirmed, we will arrange the most efficient testing services for your project immediately.
- In the meantime, we have an excellent after-sales service. If you need any other questions or concerns, you can contact us at any time.
How to Contact Us?
If you have a requirement about our services, please contact us by phone or email, our colleagues will reply to you within three working days.
Related Services




