Hybridoma Monoclonal Antibody Technology
InquiryHybridoma technology is also known as monoclonal antibody technology: In 1975, G. Kohler and C.Mistein established hybridoma technology on the basis of natural hybridization technology. They fused mouse myeloma cells that could be cultured and proliferated in vitro with pure mouse B cells immunized by antigen to become a hybrid cell line, which not only has the characteristics of tumor cells easy to proliferate in vitro indefinitely. It also has the characteristics of synthesis and secretion of specific antibodies by antibody-forming cells. The hybridoma can be cultured as a single cell to form a single cell line, that is, monoclone. Using the method of culture or intraperitoneal inoculation of mice, a large number of high-concentration, very uniform antibodies can be obtained, whose structure, amino acid sequence, specificity, etc., are consistent, and in the culture process, as long as there is no variation, the antibodies secreted at different times can maintain the same structure and function. This monoclonal antibody cannot be obtained by any other method. Due to the strong specificity of monoclonal antibody, it is widely used in biology, pharmacy, medicine and other fields, with extremely broad application prospects, so hybridoma technology for the preparation of monoclonal antibody is becoming more and more important. CD Formulation provides monoclonal antibody preparation services for mice, rats, hamsters and guinea pigs based on hybridoma technology.
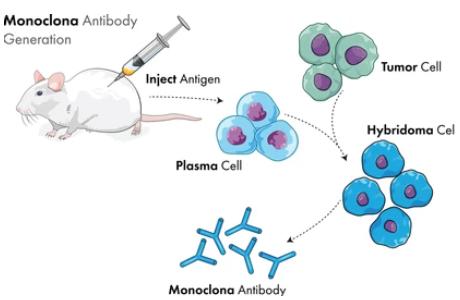 Fig.1 Hybridoma technology of monoclonal antibody production illustration.
Fig.1 Hybridoma technology of monoclonal antibody production illustration.
Advantages of Hybridoma Monoclonal Antibody
- Hybridomas can survive and be passed down "permanently" in vitro. As long as no genetic mutation occurs in the cell line, they can continue to produce highly specific and highly homogeneous antibodies.
- A large number of highly specific and uniform antibodies can be obtained using relatively impure antigens.
- Since it is possible to obtain "unlimited" amounts of uniform antibodies, it is suitable for immunological analysis methods characterized by labeled antibodies, such as IRMA and ELISA.
- Due to the high specificity and single biological function of monoclonal antibodies, they can be used for radioimmunoassay and immune-directed therapy in vivo.
Monoclonal Antibody Preparation Process
1. Selection of immune animals
Select the appropriate immunized animal based on the need to generate an immune response to the target antigen.
2. Antigen immunity
Injecting the target antigen into the immunized animal usually requires multiple immunizations to stimulate an immune response. CD Formulation offers different immune routes for customers, such as subcutaneous, intraperitoneal, intravenous, etc.
3. Cell fusion
Splenocytes or lymphocytes are obtained from immunized animals and fused with myeloma cells or other cancer cells (such as SP2/0, NS0, etc.) to form hybridoma cells.
4. Hybridoma cell screening
To screen fusion cells, HAT selective medium is generally used, and monoclonal hybridoma cells that produce the target antibody are screened out through anti-generation selection of non-hybridoma cells and monoclonal hybridoma cells.
5. Monoclonal antibody production
A single cell line is obtained from the selected monoclonal hybridoma cells and cultured and produced on a large scale. At CD Formulation, monoclonal antibodies can generally be produced in vitro or in vivo.
Related Services



 Fig.1 Hybridoma technology of monoclonal antibody production illustration.
Fig.1 Hybridoma technology of monoclonal antibody production illustration.