E. Coli Cytomembrane-Coated Nanoparticle Development
InquiryThrough our expertise in material sciences and formulation engineering, CD Formulation has generated a unique E. coli (EC) cytomembrane encapsulated nanoparticle technology. Our integrated services include membrane extraction and characterization, nanoparticle development with customizable payloads, and analytical testing to optimize delivery properties.
Why Choose EC Cytomembrane to Encapsulate Nanoparticles?
- EC is a well-characterized prokaryotic expression system capable of producing large quantities of membrane material in a well-defined and reproducible manner.
- Its cytoplasmic membrane maintains integral outer membrane proteins with conserved epitopes that can confer immunological stealth properties as well as excellent stability and compatibility with nanoparticle cores, ensuring prolonged circulation and enhanced biocompatibility.
- The presence of membrane proteins on the EC cytomembrane facilitates specific interactions with target cells, improving cellular uptake and nucleic acid delivery efficiency.
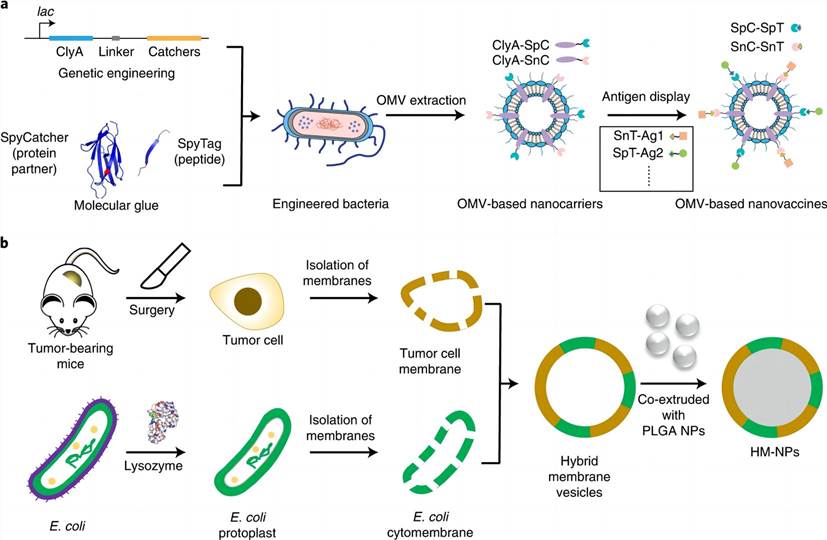 Preparation of nanoparticles based on E. coli cytomembrane (Zhao X.; et al. 2022)
Preparation of nanoparticles based on E. coli cytomembrane (Zhao X.; et al. 2022)
What Can We Do for EC Cytomembrane-Encapsulated Nanoparticle Development?
- EC Culture and Cytomembrane Extraction
Our team specializes in using cell engineering techniques to optimize EC strains to achieve higher membrane yields. We can also customize the phenotypic characteristics of strains according to customer needs, such as the expression of surface marker proteins.
We select appropriate growth conditions based on the characteristics of the strains, such as nutrient-rich media and appropriate temperatures to achieve high cell density.
Harvested EC cells are subjected to cell disruption methods such as sonication or homogenization to release the cell membrane.
Bacterial cell membranes are isolated and purified from cell lysates using membrane extraction techniques such as differential centrifugation or membrane filtration.
- Development of Nanoparticles to Carry Nucleic Acids
Factors such as molecular weight, structure, charge and stability profile are thoroughly evaluated to inform the rational design of optimized carrier systems.
Tailorable nanomaterials and formulation conditions are selected to effectively encapsulate and protect the cargo from degradation while maintaining controlled release kinetics.
Hydrophobic and electrostatic interactions are carefully modulated to fully complex and store nucleic acids within a biocompatible membrane.
- Hybrid Membrane Vesicles Development
At CD Formulation, we have developed a versatile platform for generating hybrid biomimetic vesicles through the controlled mixing of bacterial cell membranes with membranes from other sources. Our process allows for the precise incorporation of select membrane components derived from mammalian, plant or microbial cells. By modulating the ratios and compositions of the mixed membranes, we can engineer vesicles with tailored surface properties, targeting abilities, and biocompatibility profiles.
Our EC Cytomembrane-Coated Nanoparticle Analysis Services
| Parameter |
Equipment/Technology |
Content |
| Size and Dispersion |
Nanoparticle Tracking Analysis (NTA) |
Measure particle size distribution and concentration |
| Morphology |
Transmission Electron Microscopy (TEM) |
Visualize nanoparticle shape, structure and membrane coating |
| Surface Charge |
Zeta Potential Analysis |
Determine surface charge/electrophoretic mobility |
| Encapsulation Efficiency |
High Performance Liquid Chromatography (HPLC) |
Quantify the amount and purity of the encapsulated cargo |
| Release Kinetic |
UV-Vis Spectroscopy |
Monitor the in vitro release profile of the encapsulated cargo |
| Membrane Protein |
SDS-PAGE and Western Blot |
Assess membrane protein content, structure and integrity |
| Cellular Uptake |
Flow Cytometry |
Quantitatively measure nanoparticle association/internalization |
| Cytotoxicity |
MTT Assay |
Evaluate biocompatibility and cytotoxic potential |
Advantages of Our EC Cytomembrane-Coated Nanoparticle Development Services
- We offer efficient isolation of cytomembranes from various bacterial sources, including E. coli.
- We can tailor the nanoparticle core composition and size to meet specific requirements.
- Our expertise lies in coating the nanoparticle cores with bacterial cytomembranes, ensuring a biomimetic surface.
- We can engineer the cytomembranes for specific targeting by incorporating ligands or antibodies.
- We provide comprehensive characterization to ensure the quality, integrity, and functionality of the cytomembrane-coated nanoparticles.
- Our services are applicable to various fields, including drug delivery, vaccine development, and bioimaging.
Discover the potential of EC cytomembrane-coated nanoparticle development with our comprehensive services. CD Formulation specializes in isolating cytomembranes from diverse bacterial sources, enabling the biomimetic coating of customizable nanoparticle cores. Contact us today to embark on a transformative journey of using biomimetic nanoparticles to deliver nucleic acid-based therapeutics!
References
- Zhao X.; et al. Nanocarriers based on bacterial membrane materials for cancer vaccine delivery. Nat Protoc. 2022, 17(10):2240-2274.
- Long Q.; et al. Engineered bacterial membrane vesicles are promising carriers for vaccine design and tumor immunotherapy. Adv Drug Deliv Rev. 2022, 186:114321.
- Chen H.; et al. Advances in Escherichia coli Nissle 1917 as a customizable drug delivery system for disease treatment and diagnosis strategies. Mater Today Bio. 2023, 18:100543.
Related Services



 Preparation of nanoparticles based on E. coli cytomembrane (Zhao X.; et al. 2022)
Preparation of nanoparticles based on E. coli cytomembrane (Zhao X.; et al. 2022)