Dendritic Cell Development for mRNA Delivery
InquiryWith decades of experience in mRNA vaccine and cancer immunotherapy research, CD Formulation provides comprehensive services to support the design and testing of novel dendritic cell-based mRNA therapies.
How Do Dendritic Cells Deliver mRNA Vaccines?
Dendritic cells (DCs) are immune cells that have the unique ability to capture, process and present vaccine antigens to T-cells, making them a promising vehicle for mRNA vaccine delivery. Immature DCs can be harvested from patients' blood and transfected with mRNA coding for a viral or tumor protein ex vivo. The DCs efficiently translate the mRNA and present the antigen peptides via MHC molecules on their cell surface. They also mature and migrate to lymph nodes when activated. This stimulates antigen-specific T-cells and generates immune memory.
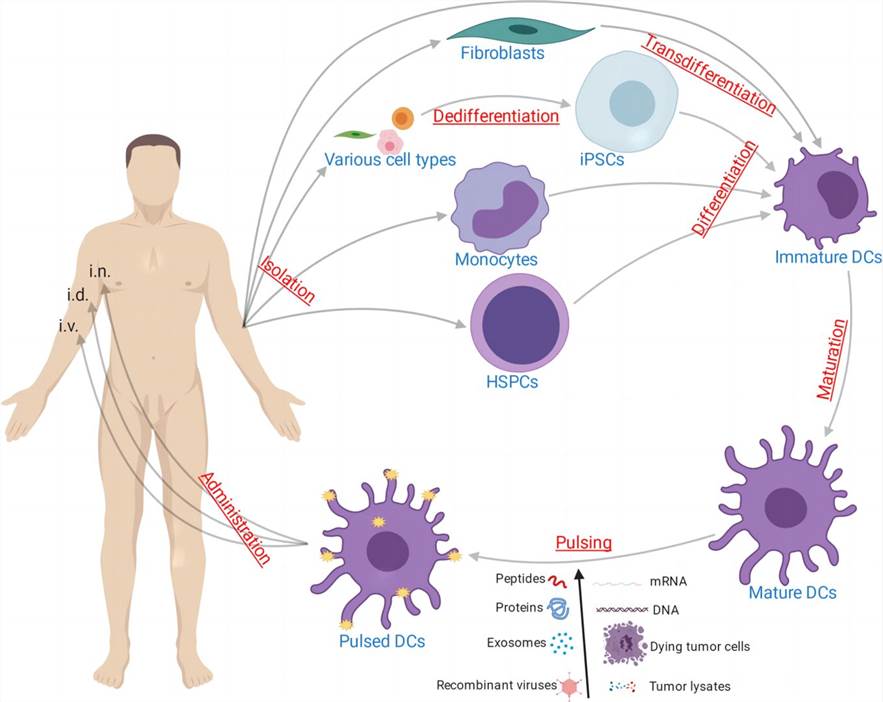 Cancer vaccine using ex vivo pulsed dendritic cells (Gu Y.Z. et al. 2020)
Cancer vaccine using ex vivo pulsed dendritic cells (Gu Y.Z. et al. 2020)
What Can We Do to Develop DCs for mRNA Delivery?
| Cell Differentiation/Transdifferentiation |
Direct Separation |
- Based on the blood or tissue samples provided by the customer, target immune cell types such as monocytes are separated from these components using selective adhesion or magnetic beads separation technology.
- For monocytes, we can directly culture them with GM-CSF and IL-4 for several days to induce their differentiation into immature dendritic cells.
- To obtain mature dendritic cells, we expose the immature dendritic cells to cytokines like TNF-α, IL-1β, IL-6 and PGE2.
|
- Dendritic cells are enriched using techniques such as density gradient centrifugation or magnetic beads-based sorting.
- Dendritic cells are labeled with specific antibodies against surface markers like CD11c.
- Labeled dendritic cells are sorted using flow cytometry or magnetic-activated cell sorting to obtain a highly pure population.
- Sorted dendritic cells are washed to remove any residual contaminants or labeling antibodies.
|
- mRNA Vaccines Development
At CD Formulation, we offer comprehensive services to facilitate the development of effective mRNA vaccines. Our expertise encompasses various stages of the process, starting with the design of mRNA sequences encoding the desired antigens or therapeutic proteins.
| mRNA Design |
We utilize advanced bioinformatics tools and knowledge of RNA biology to optimize mRNA design for enhanced stability, translation efficiency, and antigen expression. |
| mRNA Synthesis |
Our team also excels in mRNA synthesis, utilizing cutting-edge techniques to generate high-quality, modified nucleotides and ensure the production of pure and highly functional mRNA molecules. |
| Our techniques for transfecting mRNA into dendritic cells |
- Electroporation
- Cationic polymers
|
- Lipid-based transfection
- Viral vectors
|
- Calcium phosphate precipitation
- Particle-mediated delivery
|
How Do We Evaluate the Antigen Presentation Effect of DCs?
We use flow cytometry to analyze the expression of cell surface markers that indicate DC maturation and activation status, such as MHC I/II, CD80/86, CD83.
We measure the release of cytokines like IL-12, TNFα that are involved in DC-mediated T cell activation via ELISA or multiplex arrays.
- Mixed Lymphocyte Reaction
DCs are co-cultured with allogeneic T cells to assess their ability to stimulate T cell proliferation as a measure of antigen presentation.
Advantages of Our Dendritic Cell Development Services for mRNA Delivery
- Our team possesses deep knowledge of mRNA design, ensuring the incorporation of suitable modifications and codon optimization to maximize antigen expression levels in DCs.
- We employ advanced transfection methods to achieve efficient and reliable delivery of mRNA to DCs.
- Our services are customized to target specific DC subsets, optimizing delivery strategies that align with their characteristics and antigen presentation capabilities.
- We incorporate advanced methodologies to modulate co-stimulatory molecule expression on DCs, enhancing their immunostimulatory capacities.
CD Formulation provides customized approaches tailored to specific DC subsets, catering to the needs of the pharmaceutical and scientific research sectors. If you are seeking high-quality dendritic cell development services for mRNA delivery, please contact us for further information!
References
- Gu Y.Z. et al. Ex vivo pulsed dendritic cell vaccination against cancer. Acta Pharmacol Sin. 2020, 41(7): 959-969.
- Firdessa-Fite R.; Creusot R.J. Nanoparticles versus dendritic cells as vehicles to deliver mRNA encoding multiple epitopes for immunotherapy. Mol Ther Methods Clin Dev. 2019, 16:50-62.
Related Services



 Cancer vaccine using ex vivo pulsed dendritic cells (Gu Y.Z. et al. 2020)
Cancer vaccine using ex vivo pulsed dendritic cells (Gu Y.Z. et al. 2020)