CAR-T Cell Therapy Development
Inquiry
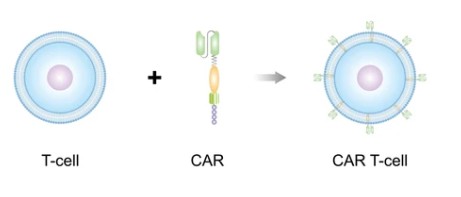
CAR-T cell therapy is a revolutionary approach to cancer treatment that combines cell therapy, gene therapy, and immunotherapy to use a patient's own T cells to fight cancer, and it is this potential to harness our immune system to fight cancer that makes CAR-T cell therapy revolutionary and has earned it the title of "living drug." But CAR-T cell therapy is a complex process that should be done by experts with extensive experience, and to advance your CAR-T cell research. CD Formulation's CAR-T cell service provides customized pre-clinical CAR-T cells generation and validation services including monoclonal antibody sequencing, chimeric antigen receptor cloning, lentivirus packaging, mouse or human T cell transduction, and quality control of CAR-T cells.
Our Services
CAR is the core component of CAR-T. CD Formulation has successfully built a professional antibody preparation technology platform with years of research and development experience, which relies on the four antibody platforms of phage display, hybridoma, FACS single B cell, and single B cell to provides different solutions for scFv discovery and efficient screening of CAR candidate molecules, accelerate the process of CAR-T research.
The extracellular domain of a CAR is usually a scFv segment of a monoclonal antibody antigen-binding region, which determines whether CAR-T can specifically recognize tumor cells and plays an important role in the efficacy and safety of CAR-T cells. Through our scFv antibody library construction platform, scFV sequences can be obtained directly and high-throughput screen CFV molecules.
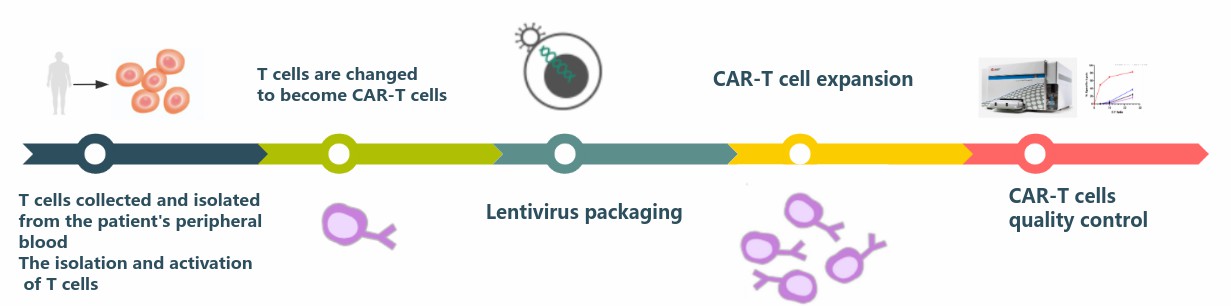
T cells collected and isolated from the patient's peripheral blood need to be activated before they can be used for modification. We have developed GMP-grade monoclonal activating antibodies with high purity, high activity, and ultra-low endotoxin, which have been validated at the cell level to effectively activate cultured T cells and better facilitate the development and production of CAR-T cell therapeutics.
After the isolation and activation of T cells is completed, lentiviral packaging is a key part of CAR-T cell preparation, and lentiviral titer is a key factor in determining the positive rate of CAR transfection. After constructing a single stranded variable fragment (ScFv), our expert technical team transfers it into a selected CAR lentiviral vector, makes the lentivirus and transduces activated human (or mouse) T cells. After the CAR-T cells proliferate, the cytotoxicity is measured in a real time assay, CAR expression analyzed and cytokine production quantified.
- CAR-T Cell Quality Control
The quality of CAR-T cells directly affects the clinical treatment effect and the health of patients, and even endangers the lives of patients if they are not rigorously tested. Therefore, CAR-T cells need to undergo rigorous release testing before they are reinfused into patients. CD Formulation provides comprehensive CAR-T cell testing services:
- Cell Viability: Detecting cell viability
- Cell Population Profiling: Monitoring CAR-T cell differentiation, exhaustion, apoptosis, etc
- Detection of Target Cells: Understanding key features of CAR-T products
- Detection of Non-Target Cells: Detecting percentage of residual tumor cells
- Test of Impurities: Detecting residual cytokines
About CD Formulation
CD Formulation provides integrated innovation solutions from strategy to implementation to help you increase opportunities and address challenges. With world-class expertise in CAR-T development, our service team can complete CAR-T projects in a relatively short time. If you have any CAR-T requirements, please contact us by phone or email, our colleagues will reply to you within three working days.
Related Services




