Biological Activity (Potency) Testing Services
InquiryCD Formulation have a wealth of experience performing potency testing to support development, transfer/validation and commercial release and stability testing of various bio-pharmaceutical products.
Why to Perform Biological Activity (Potency) Testing?
Bioassays are essential for the development of new drugs. In particular, per regulatory requirement, they are performed to determine the biological activity (potency) of the product, a critical quality attribute according to ICH Q6B, using mode of action assays.
Our Methods for Biological Activity (Potency) Testing
Potency testing involves comparison of a product's biological activity to that of a reference preparation and cell-based potency assays are central tools used to measure drug efficacy during potency testing. They allow scientists or researchers to see how a particular dose of a drug will react in a given biological system. Biological potency testing can be divided into two methods, including in vivo potency testing and in vitro potency testing.
In vivo testing uses whole animals as living organisms, which can reflect the way drugs work on the human body. It is the most classic biological potency testing method.
The conventional methods for in vitro potency testing include the tube-disc method and the turbidimetric method.
Our Services for Biological Activity (Potency) Testing
CD Formulation will develop personalized proposals to perform biological activity and potency testing for bio-pharmaceuticals based on the customer's needs. We can provide biological activity and potency testing services for a series of bio-pharmaceutical products, including but not limited to
- Monoclonal Therapeutic Antibodies
- Bispecific Monoclonal Antibodies
- Antibody Drug Conjugates (ADC)
- Complex Antibody like constructs
- Proteins/Peptides
- Vaccines
- mRNAs
- Gene and Cell Therapeutics (ATMPs)
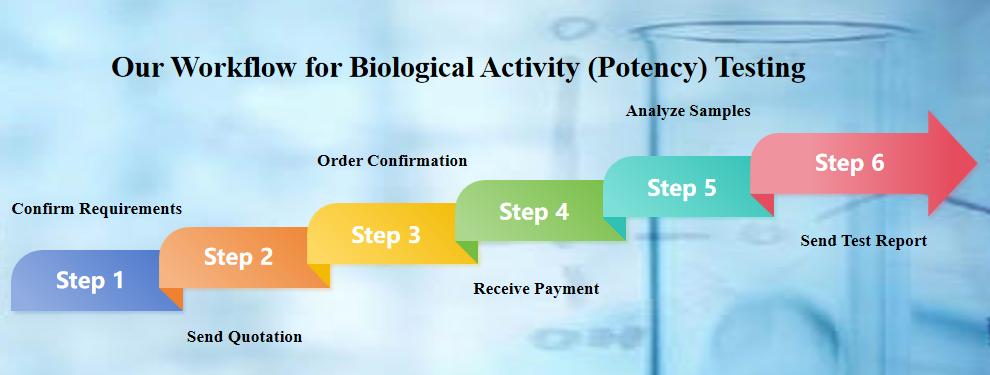
Our Workflow for Biological Activity (Potency) Testing
Here are following six steps to easily solve your requirements.
Advantages of Our Services
- Our technicians have lots of experience in potency testing, and we can provide you with the professional proposal.
- We will arrange the most efficient testing services for your project once the order is confirmed.
- We have an excellent after-sales service to solve your any issues.
How to Contact Us?
If you have a requirement about our services, please contact us by phone or email, our colleagues will reply to you within three working days.
Related Services




