Antibody-Modified Immunoliposome Development
InquiryAntibody-modified immunoliposomes are a very promising tool for targeted drug delivery. CD Formulation delves into immunoliposomes, a system in which the liposome safely delivers and releases its drug payload right where it needs to go, while the antibodies act like a homing device guiding the nanoparticle to the right targets. Our team has rich experience and expertise and can provide you with a series of services ranging from lipid material selection, antibody conjugation, drug loading optimization to in vivo and in vitro drug efficacy evaluation.
What are Immunoliposomes?
Antibody-modified liposomes, referred to as immunoliposomes, represent an advanced nanoparticulate drug carrier system achieved through the rational design and engineering of antibody-modified liposomes. As colloidal vesicles comprised of a lipid bilayer membrane, liposomes can encapsulate therapeutic and diagnostic agents within an aqueous core. Surface functionalization with antibodies endows immunoliposomes with ligand-mediated targeting capabilities. The resultant immunoliposomes exhibit synergistic properties that combine the key advantages of both liposomal technology and antibody targeting.
How Do We Prepare Immunoliposome?
Our immunoliposome development involves a meticulous process that ensures the successful integration of the desired monoclonal antibodies onto the liposomal surface. CD Formulation has perfected this process, leveraging their expertise and cutting-edge technologies to deliver high-quality immunoliposomes.
Lipids are selected based on their physicochemical properties, such as stability, fluidity, and surface charge. At CD Formulation, various methods, including thin-film hydration, sonication, or extrusion, are employed to generate liposomes of desired size and characteristics.
Various strategies are employed by us for antibody conjugation to the liposomal surface. For commonly used methods, non-covalent attachment involves the interaction between thiolated antibodies and maleimide-containing liposomes, while covalent methods utilize reactive functional groups on the lipids and antibodies for stable bond formation.
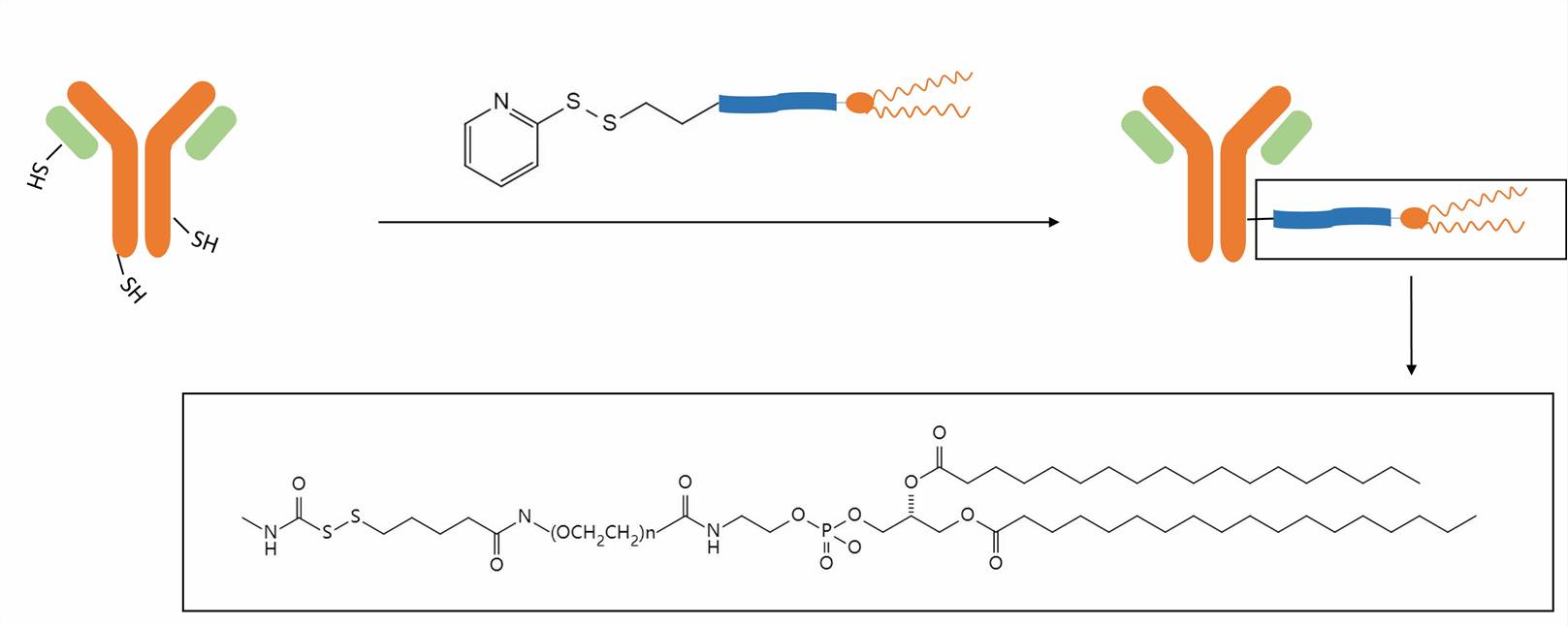
We introduced maleimide groups and thiol groups into liposomes and antibodies respectively, and the two reacted to form stable thioether bonds. This strategy has several advantages, including simplicity, mild reaction conditions, and preservation of antibody functionality.
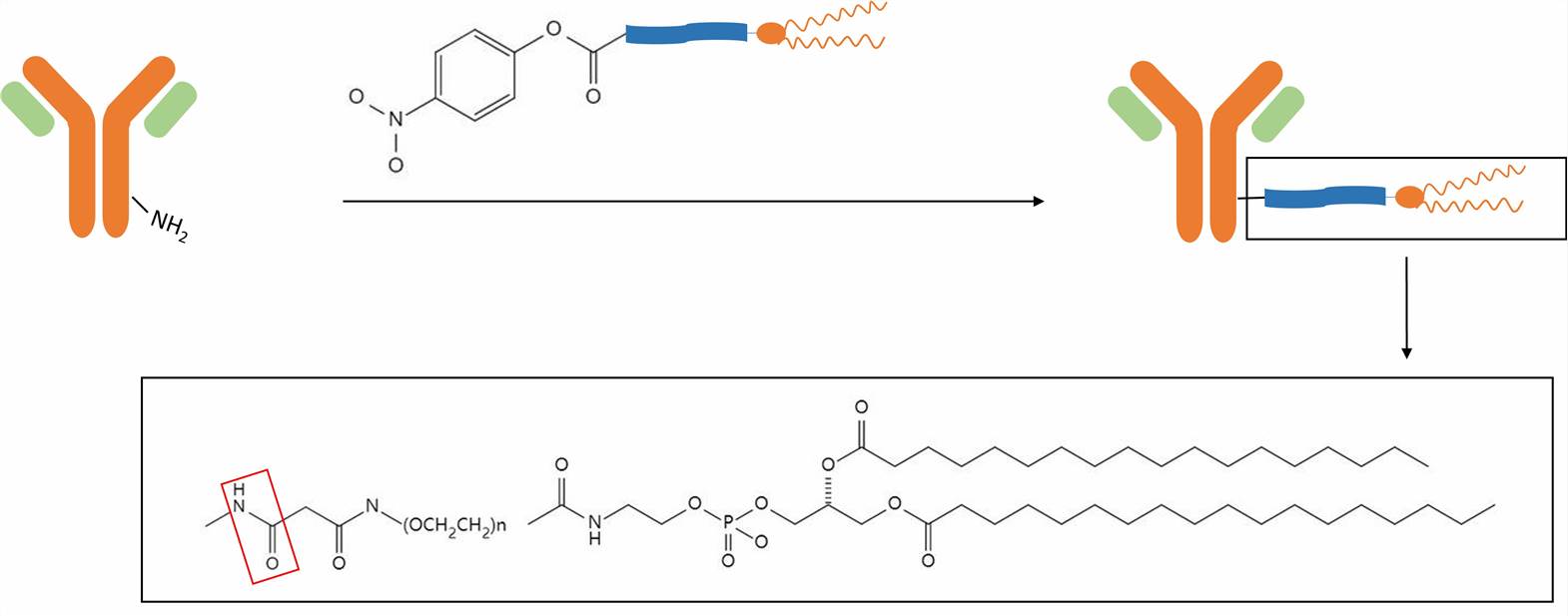
CD Formulation also uses covalent methods for antibody conjugation to ensure strong and durable binding. For example, the amino and carboxyl groups of antibodies and lipids are used to form stable amide bonds. We carefully control reaction conditions, including pH and reagent concentration, to optimize coupling efficiency and minimize side reactions.
- Characterization and Optimization
Rigorous characterization techniques are employed to evaluate the physicochemical properties of the immunoliposomes, including size, surface charge, antibody density, and stability.
- In vitro and In vivo Evaluation
CD Formulation conducts comprehensive in vitro and in vivo studies to assess the performance immunoliposomes. These evaluations include cell uptake studies, biodistribution analysis, and efficacy assessments in relevant disease models.
Applications of Immunoliposomes
CD Formulation has been actively exploring and advancing the applications of antibody-modified immunoliposomes across different disease indications.
- Infectious Diseases
- Inflammatory
- Autoimmune Diseases
- Neurodegenerative Diseases
- Cancer
Highlights of Our Immunoliposome Development Services
- Efficient drug delivery
- Enhance drug targeting
- Protect drug activity
- Achieve controlled release of drugs
- Reduce costs
Antibody-modified immunoliposomes have revolutionized the field of targeted drug delivery, offering precise and selective treatment options for various diseases. By developing immunoliposomes, CD Formulation can enhance the efficacy and targeting of drugs while minimizing off-target effects. For more information on CD Formulation's immunoliposome development services, please feel free to contact us and explore how our innovative formulations can address your specific needs.
References
- Di J.; et al. When liposomes met antibodies: Drug delivery and beyond. Adv Drug Deliv Rev. 2020, 154-155:151-162.
- Eloy J.O.; et al. Immunoliposomes: A review on functionalization strategies and targets for drug delivery. Colloids Surf B Biointerfaces. 2017, 159:454-467.
- Kirpotin D.; et al. Sterically stabilized anti-HER2 immunoliposomes: design and targeting to human breast cancer cells in vitro. Biochemistry. 1997, 36(1):66-75.
Related Services




