Analytical Method Validation of Bioactivity and Potency
InquiryCD Formulation will develop personalized proposals to perform the analytical method validation of bioactivity and potency for bio-pharmaceuticals based on the customer's needs.
Why to Perform Analytical Method Validation of Bioactivity and Potency?
Biological activity and potency measurement mainly refers to relative potency measurement. This method compares the biological reaction of the test product with the reaction produced by the known standard product, thereby quantitatively determining the potency of the test product relative to the standard product.In the quality control of biological products, bioactivity and potency is the critical quality attributes that reflects the effectiveness of biological products. Therefore, standardized methodological verification of the corresponding measurement methods is a prerequisite to ensure their applicability.
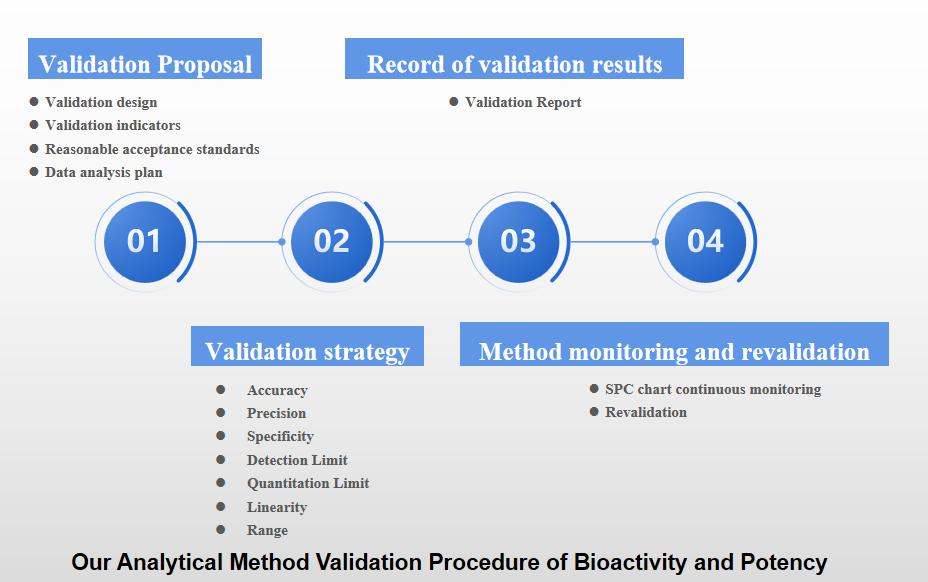
Our Analysis Method Validation Procedure of Bioactivity and Potency
Here are following four steps for analysis method validation procedure of bioactivity and potency to easily solve your requirements.
Our Services for Analytical Method Validation of Bioactivity and Potency
CD Formulation can provide one-stop services of the analytical method validation of bioactivity and potency for bio-pharmaceuticals, including validation proposal (validation design, validation indicators, reasonable acceptance standards and data analysis plan, etc.) record of validation results, validation strategy, method monitoring and re-validation.
Advantages of Our Services
- Our technician team have many years experience in the analytical method validation of bioactivity and potency for bio-pharmaceuticals. And we can provide the customers with the professional proposal to meet the customer's analytical needs.
- We have established a complete and efficient demand communication mechanism. Our team can respond to your inquiry in a timely manner after receiving your demand and develop a professional service plan for you. And we will explain the complete service process to you so that you can keep abreast of the project progress.
- We have an excellent after-sales service, when you need any other assistance, please feel free to contact us.
How to Contact Us?
If you have a requirement about our services, please contact us by phone or email, our colleagues will reply to you within three working days.
Related Services




